INTRODUCTION
Kelp (Laminariales) population dynamics are driven by a complex combination and interaction of abiotic and biotic factors such as wave disturbance, temperature, light, competition, and grazing (Dayton et al. 1984, Schiel and Foster 2015, Young et al. 2016). Broader biogeographical distributions of kelps are largely influenced by temperature (Lüning and Freshwater 1988, Muth et al. 2019) and kelps’ occupation of multiple niches within their distributions is facilitated by adaptive traits (Starko et al. 2020). Competition among kelps within fundamental niches influences benthic assemblages (Dayton et al. 1984, Clark et al. 2004, Edwards and Hernández-Carmona 2005) and due to the importance of kelps as a foundation species for these assemblages (e.g., Graham 2004), persistent and diverse kelp populations have been indicators of good ecosystem health (e.g., Christie et al. 2009, Starko et al. 2019).
Kelp persistence can be defined as the multigenerational presence of kelp within a habitat patch above a self-sustaining abundance and can be characterized by multiple factors including fecundity, recruitment, longevity, stability of a kelp patch, competitive strength, resistance to perturbation, and recovery from perturbation (resilience) (Dayton et al. 1984, 1992, 1999). These persistence characteristics interact with stochastic influences, such as those affecting spore dispersal (e.g., Gaylord et al. 2012) and fluctuating community patterns associated with cyclical environmental patterns (e.g., Pacific Decadal Oscillation, El Niño Southern Oscillation - hereafter referred to as El Niño), to influence population and community dynamics (Tegner and Dayton 1987, Edwards and Hernández-Carmona 2005, Pfister et al. 2018). Kelp persistence and kelp longevity are not tautological (though longevity can play a role as a persistence characteristic) (Frank 1968, Dayton et al. 1984) as persistence can be characterized for both annual species (e.g., Nereocystis luetkeana (K. Mertens) Postels & Ruprecht; hereafter referred to as Nereocystis) (Springer et al. 2010) as well as perennial species (e.g., Pterygophora californica Ruprecht; hereafter referred to as Pterygophora) (De Wreede 1986). As kelp population dynamics are influenced by abiotic and biotic factors, kelp persistence can be bounded by thresholds set by those factors (or their interaction) that when surpassed can result in entire ecosystem shifts (Graham 2004, Filbee-Dexter and Wernberg 2018, Rogers-Bennett and Catton 2019, McPherson et al. 2021). Furthermore, the inherent complexity of marine systems and ecophysiological differences among kelps (Muth et al. 2019, Starko et al. 2019) has led to persistence differences among kelps in the face of exacerbating perturbation events, such as for northeastern Pacific kelps such as Nereocystis, Macrocystis pyrifera (Linnaeus) C. Agardh (hereafter referred to as Macrocystis), Pterygophora, and Eisenia arborea Areschoug (previously known as Ecklonia arborea) in the advent of the 2014–2016 marine heatwave (Cavanaugh et al. 2019, Rogers-Bennett and Catton 2019, McPherson et al. 2021, Watson et al. 2021). Evidence exists for the distributional shift of kelps toward higher latitudes in the face of climate change, cumulatively being deleterious for kelp and kelp community persistence and abundance on latitudinal scales (e.g., Assis et al. 2017, Provost et al. 2017, Wernberg et al. 2019). However, habitat refugia from extreme events and climatic shifts can act as persistence buffers for kelp populations (Vega et al. 2005, Graham et al. 2007, Starko et al. 2019, Davis et al. 2021).
Though different kelps have evolved traits to occupy distinct fundamental niches including environmental conditions such as high temperature or wave exposure (e.g., Starko et al. 2019), kelps exhibit phenotypic plasticity in the presence of abiotic heterogeneity (e.g., holdfast structure for Macrocystis, Demes et al. 2009; blade structure for E. arborea, Roberson and Coyer 2004; blade structure for Nereocystis, Koehl et al. 2008). The persistence benefits provided by this plasticity has had resulting ecological (e.g., Hughes 2010), physiological (e.g., Burnett and Koehl 2019), and genetic (e.g., Fernández et al. 2021) consequences, with some ecologists suggesting consequences as great as incipient speciation (Roberson and Coyer 2004). It has long been recognized that establishing baselines for kelp plasticity and persistence is important for detecting changes among these foundation species.
Detecting change among subtidal kelp populations requires intensive methodologies due to the inherent logistical difficulties with sampling of subtidal populations. However, relevant validated methodologies previously used in ecological studies have measured temporally predictable developmental patterns as a means of inferring population dynamics. These investigators have constructed age-based models (with associated error) to estimate age using predictors such as fish length or trunk diameter (Jones 1992, Briand et al. 2006). For example, dendrochronologists have counted tree trunk rings to determine age structure within terrestrial forests (e.g., Douglass 1909), coral ecologists have used radiocarbon and uranium-thorium dating to age corals (Bard et al. 1990), and ichthyologists have used otoliths to infer population dynamics of certain fishes (Campana and Neilson 1985). Utilizing aging methodologies to construct age structures of species is particularly useful as investigators can infer multigenerational population dynamics from these age structures using methodologies employed over a shorter and logistically manageable time-scale.
Some perennial kelps employ concentric stipe growth patterns that reflect rapid and slower seasonal growth (summer and winter, respectively) with light and dark rings similar to those detectable in tree trunks (MacMillan 1902, Harper 1977). While dendrochronology can be used to infer population dynamics for trees (e.g., Swetnam 1993), similar methodology (i.e., counting annual growth rings) has been used to infer population characteristics for perennial stipitate kelp species (e.g., E. arborea, Dayton et al. 1984; Laminaria hyperborea (Gunnerus) Foslie, Kain and Jones 1963; Pterygophora, De Wreede 1984, Hymanson et al. 1990; Ecklonia radiata (C. Agardh) J. Agardh, Wernberg 2005). Although error associated with aging perennial stipitate kelps via cortical ring counts may be limiting for detecting subtle age differences among populations (Hymanson et al. 1990), the methodology has been suggested to still be useful in determining coarser patterns in population structure (Wernberg 2005). As kelp communities are affected by environmental and biotic factors, comparing age structures in the presence of heterogeneous perturbations may yield useful insight in characterizing persistence. For example, age structure extrapolations can be coupled with density data at the time of collection to generalize persistence trends pertaining to (1) evenness among age classes (survivorship trends), (2) longevity among kelp stands, and (3) recruitment among kelp stands.
While the temperate northeast Pacific supports the greatest richness of kelps in the world (Bolton 2010), the southern limit of kelps along the Mexican Baja California peninsula is dominated by the persistence of two primary kelp competitors: Macrocystis and E. arborea (Edwards and Hernández-Carmona 2005). High seawater temperature is likely the primary driver of this distributional limitation (Matson and Edwards 2007, Muth et al. 2019). Periodic conditions associated with the El Niño (i.e., high winter wave forces and high seawater temperatures) influence the intraregional dynamics by further intensifying naturally high temperature conditions and amplifying seasonal wave force perturbations (Edwards and Hernández-Carmona 2005, Cavanaugh et al. 2019). The ecophysiological capabilities of E. arborea (i.e., temperature, wave force, and desiccation resistance) enable its persistence by strengthening it competitively and allowing the species to establish large stands throughout intertidal and subtidal habitats toward the southern edge of its range (Edwards and Hernández-Carmona 2005, Matson and Edwards 2006). As kelp diversity diminishes toward the northeast Pacific southern range edge for kelps, E. arborea influences ecosystems by providing food to important herbivores (Serviere-Zaragoza et al. 1998), providing habitat favorable to certain fishes (e.g., Sievers et al. 2016), and by increasing biological diversity (Serviere-Zaragoza et al. 2003). Despite this ecological importance, persistence characteristics of E. arborea have been scarcely studied despite this longevous foundation species playing a major role in kelp bed communities toward the southern limit of northeast Pacific kelps (Dayton et al. 1984, Edwards and Hernández-Carmona 2005).
Prior studies have shown significant morphological differences among E. arborea populations at intraregional to latitudinal spatial scales (e.g., stipe length and hollowing in the subtidal, Matson and Edwards 2006; stipe length and hollowing in the intertidal, Parada et al. 2012). Furthermore, habitat heterogeneity (i.e., differences in water motion) has been shown to consequentially result in morphological differences for E. arborea across local spatial scales (Roberson and Coyer 2004). However, the link between habitat heterogeneity, E. arborea’s morphology, and E. arborea’s persistence remains unclear. Will E. arborea’s local population persistence characteristics be heterogenous in the presence of local scale habitat heterogeneity? We hypothesized that E. arborea stands around Isla Natividad (BCS, Mexico), an island with documented spatiotemporal habitat heterogeneity (Dawson 1952, Micheli et al. 2012, Boch et al. 2018), would exhibit heterogenous persistence characteristics. We derived an age prediction model using individuals from multiple E. arborea stands around Isla Natividad based on morphological characteristics that increase with age (dark cortical rings and stipe length), while also acknowledging morphological variability among individuals in the population. The prediction model was subsequently used in conjunction with non-destructive field sampling to better characterize E. arborea population persistence at six sites off the coast of the Isla Natividad, Baja California Sur, Mexico.
MATERIALS AND METHODS
Site description
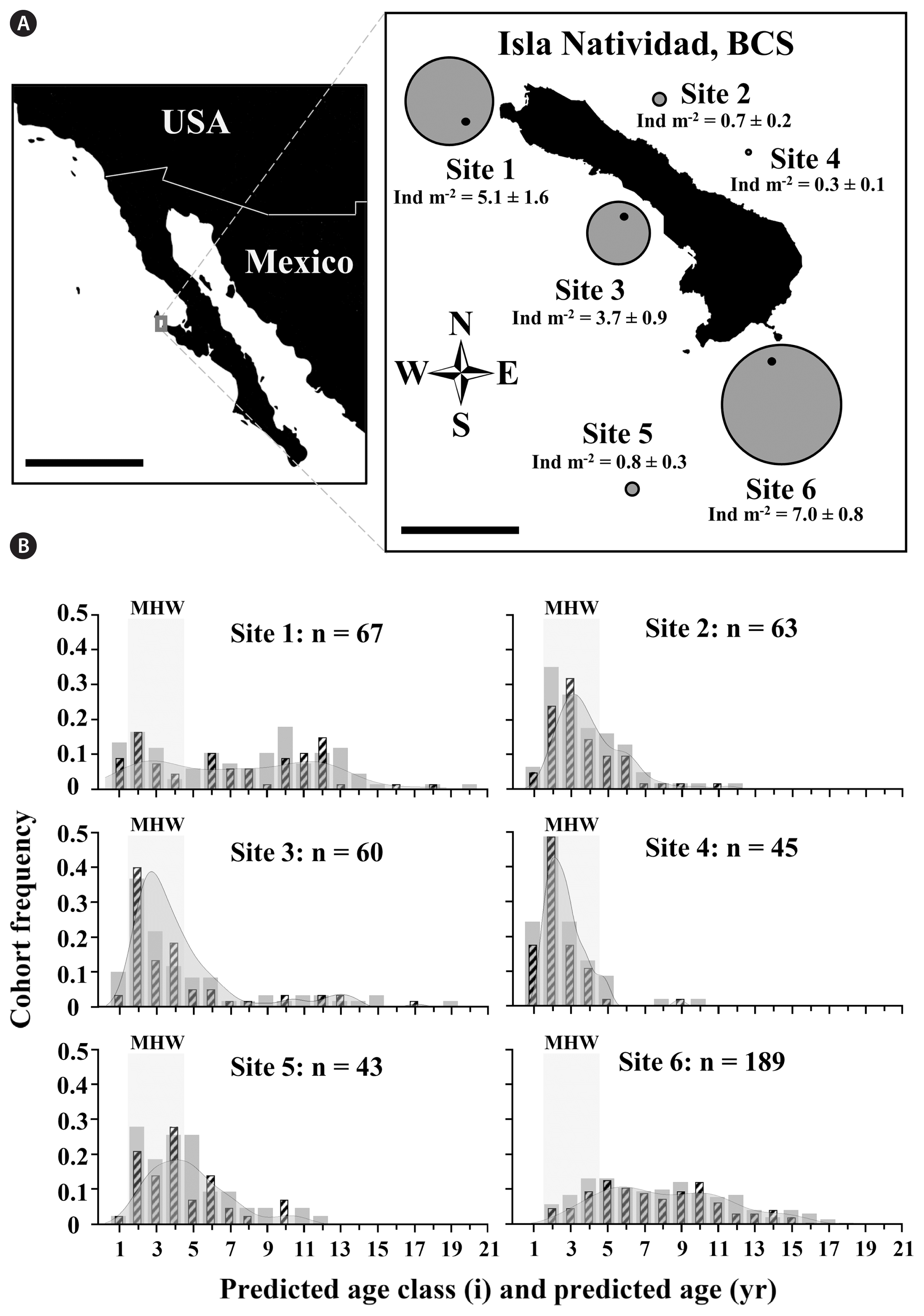
Isla Natividad is a 20 km2 island off the Pacific coast, approximately 8 km west of Punta Eugenia. Around the island, abiotic conditions are heterogenous at both island-side (East and West) and site-specific scales (Dawson 1952, Micheli et al. 2012, Boch et al. 2018). For a more detailed description of marine habitats and associated abiotic and biotic characteristics surrounding Isla Natividad, see Micheli et al. (2012), Boch et al. (2018), and Vilalta-Navas et al. (2018). Sites sampled in the current study were all within 0.5–1.5 km offshore of the island in areas with Macrocystis canopy (see Vilalta-Navas et al. 2018 regarding canopy extent). Sampled habitats were primarily rocky reef, contained few sand channels and boulder fields, and were between depths of 10–15 m. Sampling occurred between the dates of Mar 27 and Apr 2, 2018.
Field methods
To establish the age prediction model, haphazardly selected Eisenia arborea were collected (individuals separated by minimum of 2 m and a maximum of 50 m; a range of small and large individuals were selected to account for the size distribution at each site; n = 20–40 individuals per site) at four haphazardly selected sites distributed around the island (separated by 5–20 km) with Macrocystis canopy present. Entire thalli were removed from the substrate by dislodging their haptera with a dive knife and prying their holdfast from the substrate. Whole individuals were transported to the lab in mesh bags within black 200 L contractor bags to minimize sloughing or blade loss. Stipe length was measured for each individual from the most apical hapteron on the holdfast to either the closest bladelet to the holdfast for non-bifurcated individuals or the base of the bifurcation (the initial bifurcation, in the case of multiple bifurcations) (Matson and Edwards 2006). Subsequently, multiple stipe cross-sections (0.5–1.5 mm thick; 3–5 cross-sections) were taken between 5–10 cm above the holdfast and used to estimate age by counting dark growth rings (Fig. 1) (e.g., Hymanson et al. 1990 with Pterygophora). An age was estimated for each individual by quantifying the maximum dark ring count within the cortex, assuming that rings were annual (see Kain and Jones 1963, Dayton et al. 1984, De Wreede 1984, Hymanson et al. 1990). Due to no significant differences among sites in stipe length covariation with estimated age (see Results), stipe length was determined to be a feasible non-destructive field metric to obtain large sample sizes of stand age structure in-situ. Thus, in-situ stipe length measurements were used to predict age structures of E. arborea populations (see De Wreede 1984 for Pterygophora age estimation, but also see Hymanson et al. 1990 for validation concerns).
To characterize persistence of E. arborea populations around Isla Natividad, sampling of density and non-destructive sampling of stipe length were conducted at the same four sites plus two additional sites to increase sample size and number of sites (6 sites total). At each site, population density, as the number of individuals of per m2, was sampled using multiple 30 m × 2 m swaths (n = 3–9 per site based on opportunistic sampling and logistical constraints) separated by ≥10 m and at depths between 10 and 15 m. Due to the logistical constraints of limited bottom time on SCUBA and limited total time to complete the study, sampling was modified at the two sites by using shorter transects (6 m × 2 m) to estimate densities. Stipe lengths were haphazardly sampled from 20–40 individuals (separated by at least 0.5 m) along each swath upon swimming back from counting individuals per swath (a range of small to large individuals were selected to account for the size distribution at each site).
Data analysis
Statistical tests and modelling were conducted using a combination of JMP 14 and R ver. 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria). Collections of whole E. arborea individuals from stands at the initial four sites around Isla Natividad were used to test for significant differences in stipe length (fixed factor) acting as a predictor for age (response) among sites (random factor) using mixed-factor ANCOVA. As the relationship between stipe length and age did not significantly vary among sites (see Results), E. arborea stipe lengths and ages from all sites were pooled to construct a linear age prediction model with a set intercept of 0 to account for 0-year-old recruits initiating at zero stipe length. Individuals at the same site with the same estimated age were treated as subsamples to provide a single stipe length replicate associated with a single age per site. Predicted ages derived from field stipe length measurements using the age prediction model were binned in cohorts for each site (e.g., 1–2 years old). Two additional age prediction models were derived from the 95% confidence interval (CI) and used to construct a zone of error around the predicted age structures.
At each site, predicted stand age structures were constructed using the pooled model and stipe lengths measured in-situ. Population persistence was compared among sites using a combination of descriptive statistics and statistical tests assessing population age class evenness, surviving recruits, and longevity differences. Among site differences in mean predicted ages were tested using ANOVA treating site as a random factor. Significant differences in age structure distributional tails among sites were tested using Kruskal-Wallis. Pairwise predicted age structure maximum age deviations (D) were tested using Kolmogorov-Smirnov (K-S tests) with Bonferroni-Holm multiple comparisons p-value adjustments for significance (α = 0.05). Significant differences found between sites were proceeded with post-hoc adjusted K-S tests using upper or lower CI model predicted age class distributions before characterizing whether a pairwise comparison was significantly different. Each site’s predicted age structure skew and kurtosis were calculated, but not compared statistically (due to small sample size being insufficient for independent Pearson’s chi square tests). Mean predicted ages and density of surviving recruits (stand density [# · m−2] * percent within the 0–1 cohort [%]) were tested for significant differences among sites using ANOVA treating site as a random factor. Maximum age was calculated using the lower CI model for more longevous populations and the upper CI model for less longevous populations to more conservatively described island-wide maximum differences in longevity. Magnitude of effects (%) were calculated for ANOVA and Kruskal-Wallis tests to quantify the magnitude of significant differences. Eisenia arborea predicted age maxima and means within each stand were correlated with associated stand densities using Spearman’s Rank-Order Correlation. Residuals were visually assessed, and appropriate transformations were made when necessary to satisfy statistical assumptions of homoscedasticity and independence (i.e., 4th root transformation for density and estimating density of recruits).
RESULTS
No significant differences were found among sites in the covariation between stipe length and age (mixed-factor ANCOVA, F3,94 = 1.4665, p = 0.2287), allowing whole individuals collected among sites to be pooled to determine an age prediction model. We found that Eisenia arborea stipe length was a significant predictor of age (linear regression using a fixed intercept at 0 year of age, F1,23 = 61.09, r2 = 0.73, p < 0.0001) (Fig. 2) and used the derived linear age prediction model Predicted age (years) = 0 + 0.1329 (yr cm−1) stipe length (cm) and associated 95% confidence intervals (Upper - Lower = ± 0.0167 yr cm−1) as a predictor of age. Field measured stipe lengths ranged from 4 to 128 cm (n = 467) and were used to construct age structure histograms for all six sites (Fig. 3B). Densities around Isla Natividad ranged from 0.3 ± 0.1 (ind m−2; mean ± standard error) at site 4 to 7.0 ± 0.8 at site 6 (Fig. 3A). Among sites, a gradient of age structures appeared to exist ranging from right skewed (younger) and highly kurtose age class distributions with shorter lived individuals to more evenly distributed age classes with more longevous individuals (Fig. 3B). Predicted ages of E. arborea around the island ranged from 0.5 ± 0.1 to 17.0 ± 2.1 years old.
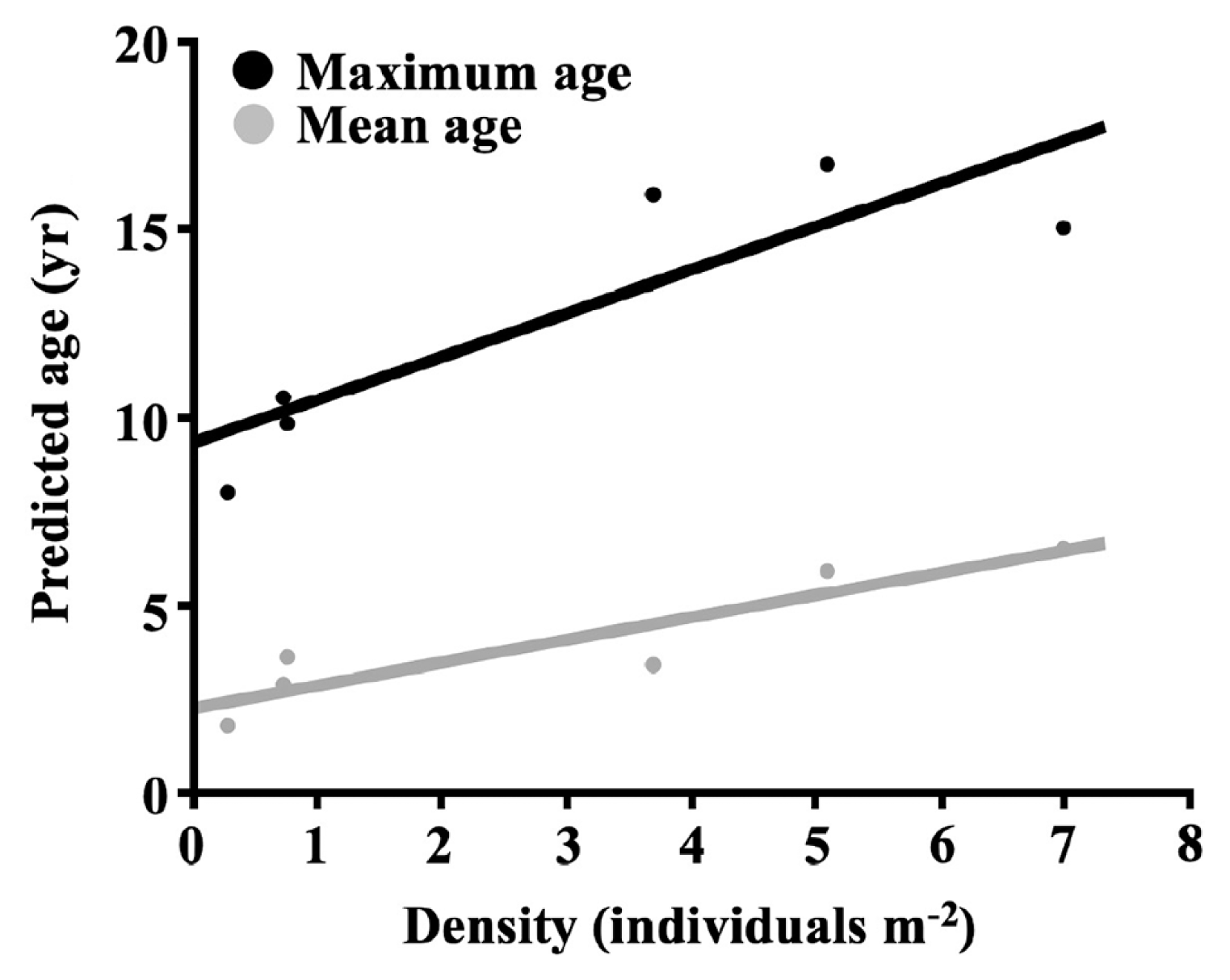
We found E. arborea mean ages among sites were significantly different but had a small magnitude of effects for those differences (one-way random factor ANOVA, F5,461 = 35.98, p < 0.0001, ω2 = 7%). The distributional tails of predicted age structures around Isla Natividad were significantly different and the calculated magnitude of effects for these differences was greater (Kruskal-Wallis, H5 = 152.00, p < 0.0001, ηH2 = 32%). The predicted age structure maximum age deviation for 73.3% of pairwise comparisons was significantly different (11 out of 15 total comparisons after post-hoc adjustment; Bonferroni-Holm multiple comparisons p-value adjustments; α = 0.05). Isla Natividad hosted E. arborea populations with wide ranging skewness (0.19 to 2.48) and kurtosis (−1.06 to 9.38) parameters for predicted age structures. Furthermore, density of recruits per site (proportion of individuals predicted to be within the 0–1 age cohort * site density) was significantly different among sites with a large magnitude of effects (one-way random factor ANOVA, F4,10 = 23.75, p < 0.0001, ω2 = 60%). Island-wide maximum longevity was heterogeneous and ranged from a 17–18-year maximum age at the oldest site (with the lower confidence interval model; site 1) to almost half the maximum age at the youngest site (9–10 with the upper confidence interval model; site 4). We also found that densities varied significantly among sites with a large magnitude of effects (one-way random factor ANOVA, F5,19 = 25.79, p = 0.0001, ω2 = 62%) and that both predicted mean age and predicted maximum age were positively correlated with stand density around Isla Natividad (Spearman’s correlation: ρ4 = 0.94, p = 0.010; Spearman’s correlation: ρ4 = 0.77, p = 0.072) (Fig. 4).
DISCUSSION
Though we found the perennial Eisenia arborea around Isla Natividad to be exceptionally long-lived, local persistence characteristics, derived from predicted age structures and including longevity, varied among E. arborea stands. Among site E. arborea mean age and structural tail differences, pairwise age structure distributional differences, predicted recruitment differences, and large descriptive statistical ranges supported our hypothesis that persistence of E. arborea was heterogenous within and among local habitats around Isla Natividad. Though our E. arborea age prediction model was designed for sites around Isla Natividad, greater latitudinal patterns in stipe length observed by Matson and Edwards (2006) may be indicative of persistence differences across broader spatial scales. These persistence differences may also be present between exposed and sheltered intertidal sites, given that the adjacent intertidal site of Punta Eugenia, BCS, was inhabited by significantly longer-stiped E. arborea in exposed versus sheltered areas (Parada et al. 2012). Wave-exposed sites can provide refugia in times of high thermal stress (Starko et al. 2019), consequentially resulting in longevity benefits within the region. We suspect persistence characteristics of E. arborea populations deviate as a function of environmental heterogeneity (Roberson and Coyer 2004) and / or competition with Macrocystis populations (Edwards and Hernández-Carmona 2005), although other biotic or interactive biotic and environmental factors may also drive variability. Overall, deterministic influences on E. arborea populations may result in different age class patterns among (1) the presence of abiotic conditions that allow E. arborea’s competitive dominance, (2) the presence of perturbation thresholds limiting longevity, or (3) the presence of superior competitors. These influences are elaborated on henceforth in the context of our results and previous studies.
Broader biogeographical patterns of kelps are bounded by environmental conditions (e.g., Muth et al. 2019) that can influence their prevalence in a system or even prevent their establishment (e.g., Tegner et al. 1997, Edwards and Hernández-Carmona 2005, Matson and Edwards 2006, Rothman et al. 2017). Within these latitudinal bounds, smaller scale abiotic perturbation (e.g., seasonally high temperatures or increased wave exposure) can be advantageous for competitively inferior kelps with perturbation resistance, such as E. arborea (Edwards and Hernández-Carmona 2005, Watson et al. 2021). While prior studies have identified ecophysiological consequences (i.e., morphological and genetic differences) among E. arborea across local spatial scales with heterogenous environmental conditions (Roberson and Coyer 2004, Parada et al. 2012), this study expands the breadth of these local spatial differences to include more specific persistence characteristics derived from stand age structures.
Macrocystis is a globally powerful competitive dominant kelp, and has been shown to regularly outcompete E. arborea (Dayton et al. 1984, Edwards and Hernández-Carmona 2005). The positive correlation between increased E. arborea density and longevity may have manifested in areas of competitive easing where Macrocystis was more limited due to perturbations (Graham 1997, Edwards and Hernández-Carmona 2005). Though all sites possessed a Macrocystis canopy over the study period, Macrocystis was reportedly removed around the entire island following the 2014–2016 heatwave (Boch pers. comm. 2018). Considering the differences among age structures around the island, abiotic perturbation associated with the 2014–2016 heatwave may have been the underlying factor that resulted in E. arborea recruitment increases in response to the absence of Macrocystis, similar to what Edwards and Hernández-Carmona (2005) saw following the 1997–1998 El Niño. The consequences of the competitive relationship on E. arborea persistence need further study, especially as (1) perturbations for Macrocystis can occur on multiple temporal scales (e.g., seasonal or ENSO cycles), (2) the competitive relationship between the two kelps differs spatiotemporally as a function of environmental heterogeneity (Edwards and Hernández-Carmona 2005), and (3) climactic changes may be changing regional perturbations influencing the competitors’ population dynamics.
Age structure evenness among E. arborea populations around Isla Natividad presided along a spectrum ranging from (1) less even (skewed, and highly kurtose) sites (e.g., site 4) to (2) more even (non-skewed, and negatively kurtose) sites (e.g., site 6). We suspect that heterogeneity among predicted cohort age evenness may have resulted from heterogeneity in deterministic site-specific perturbations driving E. arborea’s density and survivorship on a population scale (Dayton et al. 1984, Edwards and Hernández-Carmona 2005). More specifically, whereas some sites appeared to be unaffected by the 2014–2016 heat wave (sites 1 and 6) and maintained an even age structures, other sites were comprised primarily of individuals that recruited after the heat wave (sites 2–5). Although this study provided only a snapshot of E. arborea population age around Isla Natividad, we suspect that populations that possessed greater cohort evenness likely arrived in a later stage of succession as competitive dominants in habitats of refugia from Macrocystis competition and high temperatures, whereas less even age structures reflected an earlier state of succession. The largest suspected driver of successional heterogeneity likely resulted from E. arborea perturbation thresholds being mitigated at some sites, but not others, during the 2014–2016 marine heat wave (Boch et al. 2018, Starko et al. 2019, Straub et al. 2021). The resulting competitive easing likely contributed to the skew in age structure, as previously seen following the 1997–1998 El Niño (Edwards and Hernández-Carmona 2005).
Longevity of E. arborea (the maximum predicted age of individuals) was not only different across local scales, but was also positively correlated with density. This supported two characteristics of E. arborea populations: (1) longevous populations with high survivorship can dominate due to an adaptive advantage(s) (Frank 1968, Starko et al. 2019) within the southern limits of northeast Pacific kelps (i.e., resistance to wave forces, Dayton et al. 1984; and / or higher temperature, Edwards and Hernández-Carmona 2005) and (2) longevity among stands was locally significantly different, likely due to spatial (or spatiotemporal) heterogeneity in environmental (e.g., resistance thresholds) or biotic (e.g., competition) factors that affected survivorship. The relationship between longevity and density is suggestive of synergistic habitat response facilitating dominance that leads toward more stable populations in habitats where E. arborea is more adapted (resistance to perturbation) than its competitors. A similar relationship between stipe length and density has been previously observed for L. hyperborea (Christie et al. 1998), as well as within dense Ecklonia radiata (C. Agardh) J. Agardh beds (Toohey and Kendrick 2007), and may be indicative of a more generalized pattern of deterministic influences having anywhere from facilitative to deleterious influence on kelps.
The intensity and subsequent survivorship of recruits can play an important role in age class distributions, as replenishment of age classes is inherently necessary for persistence. This study was conducted before the period of E. arborea recruitment, previously observed to occur closer to summer (Gunnill 1980, del Próo et al. 2003) versus Macrocystis recruiting year-round (Schiel and Foster 2015). We observed few juvenile Laminariales blades (morphologically indistinguishable kelps; thus excluded from the study), suggesting that all 0–1 age class E. arborea individuals were survivors from the previous year’s recruitment. It was conspicuous that some sites (such as sites 5 and 6) had limited or zero individuals in that age class. An adaptive benefit may exist between stable (even) populations prohibiting recruitment of competitive kelps within dense E. arborea stands by restricting light availability, of which more is required for competitor establishment when compared with conspecifics (i.e., deeper distributional range for E. arborea compared with Macrocystis) (Dayton et al. 1984, Spalding et al. 2003). Shading effects have been shown to enable dormancy within kelp gametophytes (e.g., Kinlan et al. 2003), and the ability of perennial kelps to replenish a dislodged conspecific within a stand with an individual that was previously in a state of arrested development has been demonstrated (Toohey and Kendrick 2007). Furthermore, there has been evidence that E. arborea gametophytes hold a competitive advantage over Macrocystis gametophytes (Christensen 2018). The competitive influence of Macrocystis gametophyte recruitment within dense E. arborea stands is likely limited by exclusion of Macrocystis sporophytes due to competition (Edwards and Hernández-Carmona 2005, Benes and Carpenter 2015), environmental limitation (e.g., wave forces and temperature) (Graham 1997, Ladah et al. 1999), or Macrocystis zoospore dispersal limitation over longer distances (Reed et al. 1988). All of these recruitment dynamics may have contributed to the stability of E. arborea populations in habitats more favorable for the perennial kelp.
The results of this study suggest that kelp persistence trends vary spatiotemporally on scales as small as local, which may have resonating effects on community dynamics. Considering E. arborea rarely is the dominant kelp across its geographic range, dense and stable (even) states, such as those exhibited at the densest sites around Isla Natividad, are likely facilitated by adapted perturbation resistance facilitating dominance in selective environments (i.e., wave swept, high temperature perturbed, or deep) (Frank 1968, Dayton et al. 1984, Edwards and Hernández-Carmona 2005, Parada et al. 2012). Despite stability of certain stands of E. arborea sampled in this study, climactic changes (e.g., increasing seawater temperature) may threaten the effectiveness of these habitat refugia (e.g., Straub et al. 2021). Climactic changes and interactive stressors have already resulted in regional persistence changes for kelps (e.g., Rogers-Bennett and Catton 2019, Watson et al. 2021) and range shifts for others (e.g., Assis et al. 2017). Local persistence heterogeneity of E. arborea may reflect persistence heterogeneity for other long-lived perennial kelp species that have habitat adaptations (e.g., to high temperature and / or wave exposure), but also experience local spatiotemporal environmental heterogeneity. Furthermore, understanding the role of refugia along biogeographic boundaries may yield insight on the effects of future climactic change.