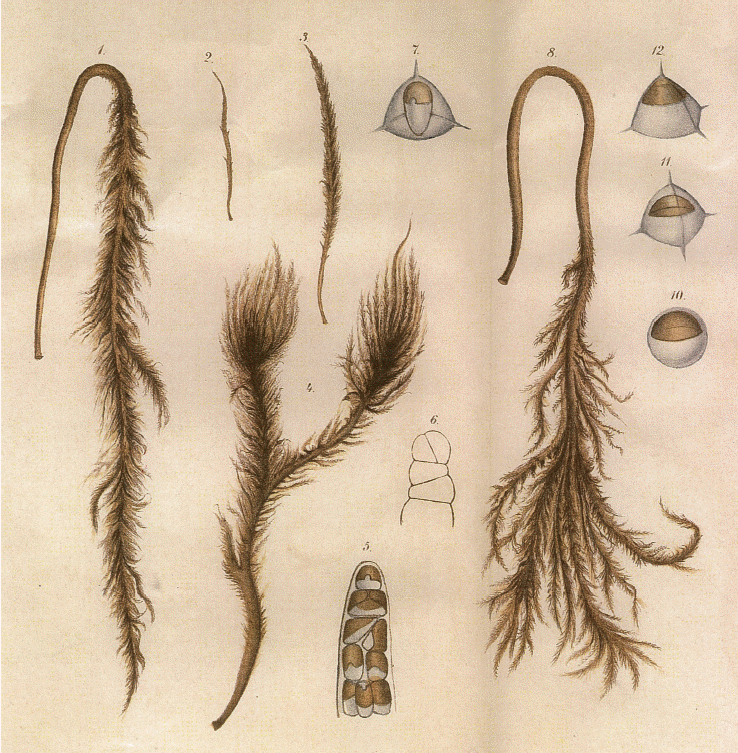Hydrurus foetidus (Villars) Trevisan is visibly a large freshwater alga found in cold rivers. The chrysophytes are better known by their planktonic members, like the colonial Chrysosphaerella or the loricate Dinobryon. In general, freshwater chrysophytes are associated with soft-water lakes, also common in dilute brown-water ponds (Wehr et al. 2015). But Hydrurus is a rheophile, preferring swiftly flowing water (Traaen and Lindstrøm 1983, Raven 1992, Rott et al. 2006a). It is also a psychrophile (sensu Cavicchioli 2016), dependent on low temperature. Hydrurus appears to be of specific importance for the early emerging aquatic larvae of chironomids (e.g., Ward 1994, Niedrist and Füreder 2018 and references therein).
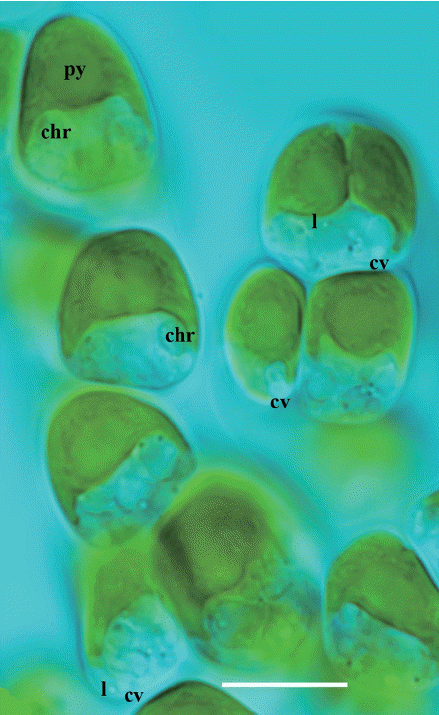
Hydrurus thrives well under seasonal climatic conditions, where there is snow in the winter, cold meltwater and moderate summer temperatures. Its early emergence at time of snowmelt makes it visually prominent–but it can be overlooked by scientists unless they know what to look for, when and where to sample and how to recognize it (e.g., Rott et al. 2006a, 2006b, Klaveness 2017, Fig. 2). As winter declines and insolation and daytime increases, Hydrurus may be the first alga visually present through the water scope. Depending upon local stream conditions and other factors still to be studied, the thalli may be richly branched (Klaveness et al. 2011) or only a central axis densely covered by small branchlets (due to “sehr starke Strömung” according to Bursa 1934, I, p. 76). Rostafiński (1882) made exact artistic representations of details and variability of this species–here reproduced as Fig. 1. Examination of samples by light microscopy (Fig. 2) reveals characteristic details, discerning Hydrurus from the emerging algal competitors. When photos of specimens sampled at different locations are compared, variants of morphological organization may be seen. More samples, more cultures and further investigations by experiments and genetic methods are required to resolve questions about phenotypic plasticity, ecotypes or species identity (cf. the genus and species in http://www.algaebase.org).
The abiotic and biotic environments from where the samples were taken should be recorded–as has been done in Austria particularly (e.g., Geitler 1927, Kann 1978, Rott and Wehr 2016 and references therein), in Norway (Lindstrøm et al. 2004, Remias et al. 2013, Rott and Schneider 2014), recently in Croatia (Koletić et al. 2017), and the classical investigations in the Tatra mountains in Poland (Rostafiński 1882, Bursa 1934, Kawecka 1989, 1993, 2003a, 2003b).
Regional surveys have shown that Hydrurus foetidus is not found in every river that may be a suitable habitat (Geitler 1927, Bursa 1934, Parker et al. 1973). Detailed studies of the rivers in the Polish Tatra (Bursa 1934, I, pp. 70–74 and 1934, II, with discussion) have revealed the temperature sensitivity of this species. An optimal range for Hydrurus in this area is given as 2–12°C, whilst higher temperature may only be tolerated for a very limited time (hours). Bursa (1934) established that Hydrurus could perish in lower ranges of rivers during the summer and therefore needed to be reintroduced from colder ranges of the river system–at higher elevations in glacial rivers, or closer to the source (cold springs) in rivers of krenal origin. Bursa (1934) distinguished between the permanent and the temporal ranges of rivers–where the lower limits of Hydrurus populations would fluctuate from year to year (Hydrurus “wanderungen”). Kann (1978) studied the entire communities of algae in her rivers, and only in “summer-cold” ranges found permanent communities of Hydrurus. Whereas, Hydrurus located in lower regions (summer-warm) might only support short-lived spring populations. In general, possible summer survival may be limited to resting stages (silicified stomatocysts), to palmellae, or to shredded central axes at shielded locations in the river, as thalli may fragment and disappear under these conditions (also noted by Canter-Lund and Lund 1995). Under moderate seasonal climates, Hydrurus may have a visible or conspicuous reappearance early in the autumn (e.g., in Austria: Kann 1978, Rott et al. 2000; in Norway: Lindstrøm et al. 2004; in Croatia: Koletić et al. 2017). It may be present through autumn and winter in some glacial streams in Switzerland (Uehlinger et al. 2010) and in the United States (Hoffman et al. 1986). During winter, Hydrurus may survive as “amorphic” (Kann 1978), while Rostafiński (1882, separatum p. 33) and again Bursa (1934) find persistent forms of Hydrurus during winter in some Tatra mountain river ranges. The waxing and waning of Hydrurus in permanent locations, or its spatio-temporal development, may be affected by seasonal or yearly challenges as flooding, nutrient availability, competition, parasitism, and grazing–often in combination with the variability of temperature and light conditions (Kann 1978, Kawecka 1989, 1993, 2003a, 2003b, Rott et al. 2006a, 2006b, Robinson et al. 2010, Rott and Wehr 2016).
When assembling and studying the printed contributions about Hydrurus foetidus, we find an apparent limit of distribution to outside of an approximate 40° N to 40° S belt around equator. Ward (1994) referred to the distribution of Hydrurus as holarctic, which also should include some cold temperate inland localities (as a high-mountain location in east Turkey at 39°44′ N) (Çevik et al. 2007). The most apparent exceptions to the distribution limits suggested above are the very high mountain observations in Nepal and Tibet / China (e.g., Hirano 1969, Subba Raju and Suxena 1979). Our preliminary conclusions are influenced by lack of observed sightings near to the 40° N latitude in south-east Asia as well as in alpine areas in the south Americas where there are positive records at 41° S (e.g., Villanueva et al. 2010).
The long-lasting winters in both South and North Korea due to areas and elevations permitting snow in abundance, makes a closer look into rivers to be of interest. As mentioned above, the summer temperature in rivers may be limiting to distribution. Among the closest published records of Hydrurus populations to Korea are in northern Japan (e.g., Kobayasi 1961, Fukushima 1962, Hara and Shitara 2009) or Russia (Primorsky region by Gontcharov 1996, Rudnaya river by Barinova et al. 2008). Observations from North Korea would increase the probability of many positive records. Korean scientists are, by this note, encouraged to visit rivers at times of snowmelt, observe, and if possible establish and study new cultures of this unique chrysophyte, since, to my knowledge, my culture is still the only one in existance. See Klaveness (2017) for environmental and culture conditions.