INTRODUCTION
Chondrus, a red algal genus, is an important primary producer providing food and habitat for various marine organisms as many seaweeds do (Lindstrom 2009, Choi et al. 2015a). This alga is also economically important as a major source of carrageenan (Necas and Bartosikova 2013, Zhou et al. 2014). However, natural populations of Chondrus have declined due to a variety of abiotic (e.g., pollution, ocean acidification, and typhoons) and biotic environmental factors, including predation by herbivores and pathogens (Correa and McLachlan 1992, 1994). Production of natural or cultivated seaweeds has been diminished by seaweed diseases caused by fungi, bacteria, viruses, and endophytic algae (Gachon et al. 2010, Potin 2012, West et al. 2013). Endophytic algae can be infectious and can negatively affect the performance of host seaweeds (Apt 1988, Correa and McLachlan 1992, 1994, Correa et al. 1997). The morphological symptoms of infection with endophytes in host seaweeds include thallus thickening, degradative lesions, cellular damage, gall formation, and morphological deformations (Yoshida and Akiyama 1979, Apt 1988, Correa et al. 1988, 1997, Garbary et al. 2014, Preuss and Zuccarello 2014). The endophytic infection may even reduce the survival, growth, and fertility of host seaweeds (Faugeron et al. 2000, Schoenrock et al. 2013).
Correa and his colleagues conducted intensive studies on endophytes in a red alga, C. crispus (Correa et al. 1988, 1994, Correa and McLachlan 1992, 1994). They found four endophytic species, including three green endophytes, Phaeophila dendroides, Endophyton ramosum (currently regarded as Ulvella ramosa) and U. operculata (previously known as Acrochaete operculata), and one brown endophyte, Streblonema species. Among these endophytes, U. operculata caused depigmentation, cellular disorganization, and wart-like protrusion in C. crispus (Correa et al. 1994). Interestingly, in the presence of U. operculata, bacteria accelerated cell wall degradation, resulting in the fragmentation and death of C. crispus (Correa et al. 1988, Correa and McLachlan 1992, 1994). As a protective mechanism, C. crispus gametophytes produced kappa-carrageenan oligosaccharides to inhibit infection of endophytic U. operculata (Bouarab et al. 2001, Weinberger 2007).
Chondrus ocellatus, which grows on semi-exposed rocky shores, is a bioengineer enhancing species diversity as primary producer and food resources of various marine organisms, and is a potential carrageenophyte in Korea (Kim et al. 2006, Zhou et al. 2014). Lee et al. (2013) reported green endophytes in C. ocellatus, without identifying the species and a green endophytic U. ramosa was found in C. ocellatus fronds by Choi et al. (2015b). We also isolated two endophytes, U. ramosa and Mikrosyphar zosterae from C. ocellatus fronds. This study aimed to examine the effects of endophytic U. ramosa and M. zosterae on the growth of their host C. ocellatus, morphological changes of the host seaweed following infection with two infectious endophytes, and the importance of apical growth tips in C. ocellatus.
MATERIALS AND METHODS
Sampling
Chondrus ocellatus samples were randomly collected from the low intertidal zone of Manripo, Taean, Korea (36°78′ N, 126°8′ E) in July 2015. The samples were put in labelled plastic bottles, placed in a cooler, and transported to the laboratory. All samples were classified into abnormal fronds with black spots (or wound, and whitening, etc.) as an indication of endophyte infection and healthy fronds with no endophyte infection.
Endophyte isolation, uni-culture, and identification
Disc-shaped samples of C. ocellatus fronds with black spots were excised using a cork borer (1 cm in diameter). The discs were rinsed for 30 s with running tap water and immersed in a 0.5% hypochlorite sodium (NaClO) solution for 30 s in order to remove microorganisms. The discs were then rinsed several times with autoclaved seawater (Gauna et al. 2009). The discs with black spots were placed in petri dishes (size: 15 mm × 60 mm) containing 10 mL of Provasoli’s enriched seawater (PES) medium (Provasoli 1968) and cultured in an incubator at 20°C and with 20 μmol photons m−2 s−1, and a photoperiod of 12 : 12 h LD cycle. Light was provided by 40 W cool-white fluorescent tubes and the level of irradiance was measured using a digital illumination meter (DX-200; INS Enterprise Co., Taipei, Taiwan). The growth of diatoms was inhibited by adding GeO2 (3 μg L−1) in culture medium during the first week of cultivation to reduce any negative effects of GeO2 (Fernandes et al. 2011). Filamentous brown M. zosterae and green U. ramosa endophytes appeared on the surface of frond discs within 3 to 4 weeks of cultivation. The endophytes were isolated, stock-cultured, and identified as M. zosterae and U. ramosa based on molecular analysis (using the RuBisCo spacer and tufA for the M. zosterae and U. ramosa analyses, respectively) and morphological features (Fig. 1A & B).
Preparation of Chondrus ocellatus juveniles
Healthy and fertile carposporophytes (>10 cm in length) of C. ocellatus were cleaned in autoclaved seawater and immersed in 1.5% KI solution for 20 min to remove surface microorganisms without releasing damaged spores (Wang et al. 2006, Li et al. 2010). The fronds were rinsed again several times in the autoclaved seawater, and several hundred frond discs bearing cystocarps were obtained using a cork borer. The discs were dried at room temperature (20°C) for 1 h and submerged in autoclaved seawater to induce carpospore release.
Carpospores were inoculated into Petri dishes containing six cut microscope slides (25 × 20 mm) and PES medium (40 mL), and then were left overnight to settle. The following day, the slides bearing sporelings were transferred to a culture dish (size: 40 mm × 100 mm) containing 100 mL of PES medium and GeO2 (3 μg L−1). Sporelings were cultured at 20°C, 60 μmol photons m−2 s−1 and 12 : 12 h LD cycle. The culture medium was replaced weekly.
Endophyte effect on the growth of Chondrus juveniles
To examine the effects of the endophytes on the growth of C. ocellatus juveniles, 48 C. ocellatus juveniles (9–11 mm in length) were prepared. They were allocated into four endophyte treatments, with each endophyte treatment having 12 C. ocellatus juveniles that were placed in plant culture dishes containing 50 mL of autoclaved seawater each. The endophyte-free treatment (control) had Chondrus juveniles only. Three mixed cultures of endophyte-M (C. ocellatus + M. zosterae), endophyte-U (C. ocellatus + U. ramosa), and endophytes-M·U (C. ocellatus + M. zosterae + U. ramosa) were also prepared. Endophyte suspensions were made by mixing the specified endophytes grown in an area of 4 cm2 and blended for 20 s with 100 mL of autoclaved seawater. Endophytic species grown in Petri dishes were carefully detached by a blade prior to make endophyte suspension. The Mikrosyphar·Ulvella endophyte suspension was prepared with U. ramosa (2 cm2) and M. zosterae (2 cm2). For each treatment, an endophyte suspension (100 mL) of M. zosterae, U. ramosa, or Mikrosyphar·Ulvella was inoculated into each plant culture dish containing 12 C. ocellatus juveniles. The control dishes with no endophyte (C. ocellatus juveniles only) were inoculated with 100 mL autoclaved seawater.
During the 1-week culture period, endophyte-M, endophyte-U, and endophytes-M·U grew and released zoospores into their respective plant culture dishes. Zoospore release from the experimental endophytes was confirmed under a light microscope using the cut glass slides that had been inserted into the cultures at the beginning of the experiment. After 1 week, 4 C. ocellatus juveniles from the 12 juveniles in each endophyte treatment group were selected and placed into three replicate beakers, each containing 100 mL of PES medium. The control dishes having 12 C. ocellatus juveniles were also treated like the other endophytic treatments and absence of endophytes was confirmed with the cut glass slides placed on the bottom of the culture dishes. After this, the samples were cultured for 3 weeks at 20°C and with 60 μmol photons m−2 s−1 and a 12 : 12 h LD cycle. The culture medium was replaced weekly.
All the experimental C. ocellatus juveniles were weekly cleaned by using a paint brush, photographed, and measured in frond length using Image J ver. 1.48 software (National Institutes of Health, Bethesda, MD, USA). We supposed that cleaning activity of the juveniles was influenced with same intensity for endophyte infection and proliferation. New proliferations were counted after 3 weeks. Relative growth rates (RGRs) of fronds (lengths) were estimated by the following formula (Kim et al. 2006):
Where Lo is the initial frond length, Lt is the final length after t days of cultivation.
Morphological symptoms of infection in host C. ocellatus juveniles were also inspected by the naked eye and under an Olympus BX53 microscope (Olympus, Tokyo, Japan). C. ocellatus tissues, either infected or healthy, were photographed under microscope after semi-thin cryosection. Prior to sectioning, samples were fixed in a 10% formalin-seawater solution and then embedding medium (Tissue-Tek O.C.T. compound; Sakura Finetek USA Inc., Torrance, CA, USA) in a cryochamber at −30°C. Sections were made with a Shandon Cryotome Cryostat microtome (Shandon-Thermo Fisher Scientific, Waltham, MA, USA) and stained with a 10% hematoxylin solution (Mayer’s hematoxylin; Dako North America Inc., Carpinteria, CA, USA).
Importance of apical growth tips of Chondrus juveniles
The endophyte infection experiments showed that U. ramosa endophytes infected selectively the apical growth tips of host C. ocellatus resulting in the reduction of the growth of C. ocellatus juveniles. Therefore, an additional experiment on the role of apical growth tips in Chondrus juveniles were designed. Twelve C. ocellatus juveniles were divided into apices (from tip to middle) and bases (from middle to base). Twelve intact juveniles, with both apices and bases were used as a control. Three replicate beakers for each treatment, containing four C. ocellatus juveniles and 100 mL of PES medium each, were prepared. The parts of C. ocellatus juveniles (apex, base, or intact) were cultured at 20°C. The irradiance and photoperiod were 60 μmol photons m−2 s−1 and 12 : 12 h LD cycle, and the culture medium was replaced weekly. Because the C. ocellatus juveniles were so small, total frond weights of each replicate (comprising four juveniles) were measured at the beginning of the experiment and after 3 weeks of cultivation. Frond RGRs (weights) of C. ocellatus juveniles were calculated using the equation shown above. The total number of new proliferations from the four juvenile fronds was also counted.
Data analysis
A one-way ANOVA was applied to evaluate the effect of endophytes on the RGRs of fronds (lengths) and the number of new proliferations in C. ocellatus juveniles. A one-way ANOVA was also used to examine the effect of apical meristems on the RGRs (frond weight) and the number of new proliferations in C. ocellatus juveniles. Homogeneity of variances was tested using Cochran’s test. Tukey’s honest significant difference (HSD) test was applied when significant differences between means were detected (Sokal and Rohlf 1995). Statistical analyses were carried out using STATISTICA ver. 7.0 software.
RESULTS
Symptoms in the infected Chondrus
C. ocellatus juveniles grew well for 3 weeks, without morphological changes (Fig. 2A–C), in the absence of the brown endophyte M. zosterae. When cultured with M. zosterae, host C. ocellatus formed black spots on their fronds except for apical areas (Fig. 2D). C. ocellatus also developed many wart-like spots (Fig. 2E) where endophytic filaments penetrated the cortex of C. ocellatus to the upper medulla zone (Fig. 2F). The apical tips of C. ocellatus juveniles were coated by U. ramosa endophytes (Fig. 2G), and dark green patches were found on the surfaces of whole host fronds (Fig. 2H). This green U. ramosa endophyte was also found in host tissues (Fig. 2I). In the endophytes-U·M treatment, green patches and wart-like spots were observed on the C. ocellatus fronds (Fig. 2J & K). Endophytic filaments of both species, U. ramosa and M. zosterae, were also observed on fronds and in host tissues (Fig. 2K & L).
Effects of two endophytes on Chondrus growth
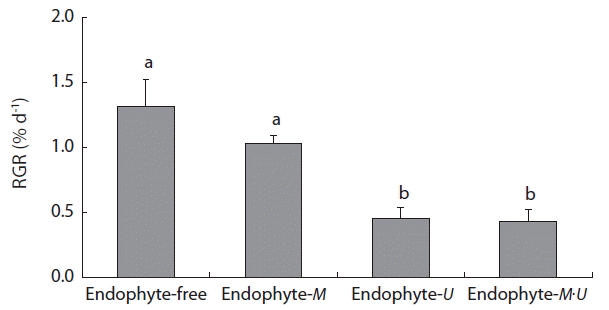
Chondrus ocellatus juveniles grew with infectious M. zosterae or U. ramosa, or with both. In 3 weeks of culture after infection, the RGR was significantly greater in the endophyte-free and endophyte-M treatment groups than in the endophyte-U or endophytes-M·U treatment groups (Fig. 3). Interestingly, no differences were found between the endophyte-free and endophyte-M treatment groups, and between the endophyte-U and endophytes-M·U treatment groups (Fig. 3), which indicates that the significant negative effects on the growth of host C. ocellatus juveniles were attributable to infection with U. ramosa.
Three weeks after infection with the endophytes, no C. ocellatus bladelets developed in the endophyte-free and endophyte-M treatment groups, 1.33 ± 0.33 (mean ± standard error [SE], n = 3) bladelets formed in the endophyte-U treatment group, and 1.67 ± 0.66 (n = 3) bladelets formed in the endophytes-M·U treatment group. The number of newly formed bladelets appeared to be significantly greater in the endophyte-U and in the endophytes-M·U treatment groups as compared to the control (endophyte-free) or endophyte-M treatment groups (p < 0.05). However, no significant differences were found between the endophyte-free and endophyte-M treatment groups and between the endophyte-U and endophytes-M·U treatment groups.
Inportance of apical meristems in Chondrus juveniles
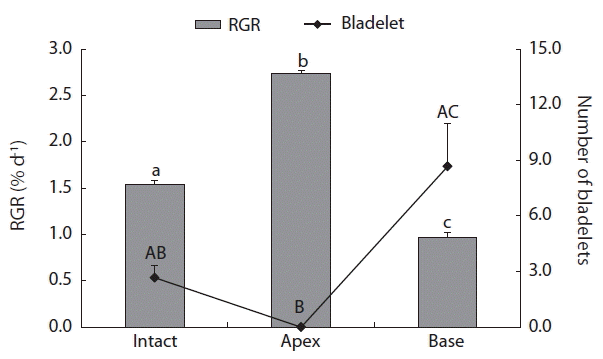
After 3 weeks of culture, the RGR values (frond weights) of C. ocellatus juveniles ranged from 0.97 to 2.73% d−1 (Fig. 4). The values were significantly different between the three treatments, with the highest RGR value in the apex treatment and the lowest in the base treatment (Fig. 4). In addition, the number of newly formed bladelets was significantly different between the treatment groups (p < 0.05). A Tukey’s test revealed a significant difference between the apex and base groups, but no differences were found between the intact and apex groups, and between the intact and base groups (Fig. 4). Intact juveniles of C. ocellatus produced a few bladelets (Fig. 5A), whereas the apex alone produced no bladelets (Fig. 5B). However, the base of C. ocellatus juveniles produced many bladelets in the excised, lateral parts of fronds (Fig. 5C). The number of newly formed C. ocellatus bladelets was 2.67 ± 0.67 (mean ± SE, n = 3) in the intact control and 8.67 ± 2.33 (n = 3) in the base treatment group. This result indicates that C. ocellatus juveniles grow apically, but that their growth can switch to bladelet production in the absence of apical meristems.
DISCUSSION
Previous studies have shown that C. crispus harbors endophytes such as brown Streblonema spp. and green U. ramosa and U. operculata (Correa et al. 1988). Choi et al. (2015b) recently isolated a green endophyte, U. ramosa, from C. ocellatus. Here, we report that the brown endophyte M. zosterae was present in C. ocellatus. This is the first report of M. zosterae as an endophyte of a carrageenophyte. We also found that morphological symptoms in C. ocellatus of infection with the two endophytes, green U. ramosa and brown M. zosterae, were different. U. ramosa infection produced dark green patches, whereas M. zosterae infection resulted in wart-like spots. Various green endophytic species in several carrageenophytes induce color changes, form holes, or have other effects on host plants (Table 1).
Although endophytes can cause some morphological changes in seaweeds, the causative organisms forming galls (hyperplasia and hypertrophy in host seaweed cells) on red algal fronds were found to be mostly bacteria, fungi, animals, or viruses (Apt and Gibor 1991, Correa et al. 1993, Goecke et al. 2012). On C. crispus fronds, galls formed in response to nematodes and bacteria (Apt 1988). Another red alga, Iridaea laminarioides (currently regarded as Mazzaella laminarioides) also developed galls on its fronds because of infection with the cyanobacterium Pleurocapsa sp. (Correa et al. 1993). The findings in the present study indicate that infection with the endophyte M. zosterae can also result in the formation of gall-like structures in the red alga C. ocellatus.
In the present study, the infectious endophytes inhibited the growth of host C. ocellatus juveniles, but the degrees of negative effects were different between the endophytes, M. zosterae and U. ramosa. C. ocellatus juveniles grew significantly better in the endophyte-free (1.32% d−1 in RGR) and endophyte-M treatment (1.03% d−1) groups than in the endophyte-U (0.45% d−1) and endophytes-M·U treatment (0.43% d−1) groups. In comparison to the RGR values with endophyte-free treatment, the RGR of the host C. ocellatus was reduced by approximately 70% with endophyte-U treatment. In this condition, U. ramosa densely covered the apical meristem tissues of C. ocellatus, perhaps reducing photosynthetic activity.
This study is the first report that the endophyte U. ramosa damages apical meristem tissues of C. ocellatus juveniles. The growth of C. ocellatus was mainly apical, and transverse divisions in apical cells are common. Apical division of C. ocellatus was not observed in the endophyte-U treatment or endophytes-M·U treatment groups (Fig. 2). This resulted in a significant reduction in the RGRs in these conditions. Removal of apical meristems from C. ocellatus juveniles reduced growth by 37% compared to that in the intact treatment group and by 65% compared to that in the apex treatment group. This result implies the importance of apical meristems to the growth of C. ocellatus. Previously, C. crispus were shown to grow three times more in apical meristems than in other parts of the organism (Chopin and Floc’h 1992).
Interestingly, defects in the apical meristems of C. ocellatus resulted in the formation of bladelets. Surface and marginal proliferations are commonly observed in adult-stage reproductive organs in Chondrus species, including C. nipponicus and C. ocellatus (Brodie et al. 1991, 1993). In this study, we found that C. ocellatus, even in the juvenile stage, produced small bladelets (≤2.1 mm in length) in the absence of apical growth tips or with apical tissue damaged by U. ramosa. Formation of many bladelets in the damaged fronds or in the basal fronds of C. ocellatus may be a mechanism to compensate for the lack of growth in the removed apical meristems. Similarly, Fucus vesiculosus produced many lateral branches that served as new apical meristems when their apices were damaged (Honkanen and Jormalainen 2002). Chondrus crispus was also shown to produce many bladelets (Corey et al. 2012). Corey et al. (2012) reported that bladelet development in C. crispus was more pronounced at temperatures of 17°C or above, and high temperatures may be optimal for the growth of the green endophyte U. operculata or U. ramosa, or both (Correa et al. 1988).
Here, we reported M. zosterae as a new endophyte of C. ocellatus. What we do not know is the level of host-specificity of M. zosterae. Although M. zosterae formed wart-like spots on C. ocellatus, this endophyte did not cause significant negative effects on the growth of the host alga. Therefore, there is a need to analyze more seaweed species, especially carrageenophytes to determine the host-specificity of M. zosterae. It is also important to determine whether different life stages of C. ocellatus respond differently to these endophytes, because some endophytes (e.g., U. operculata) affect the sporophytic fronds of C. crispus more severely than its gametophytes (Correa and McLachlan 1994). The endophytes found in this study, M. zosterae and U. ramosa, are pathogens of C. ocellatus that result in distinctive symptoms, and, in the case of U. ramosa, decrease growth of the host seaweed by damaging meristem tissues.