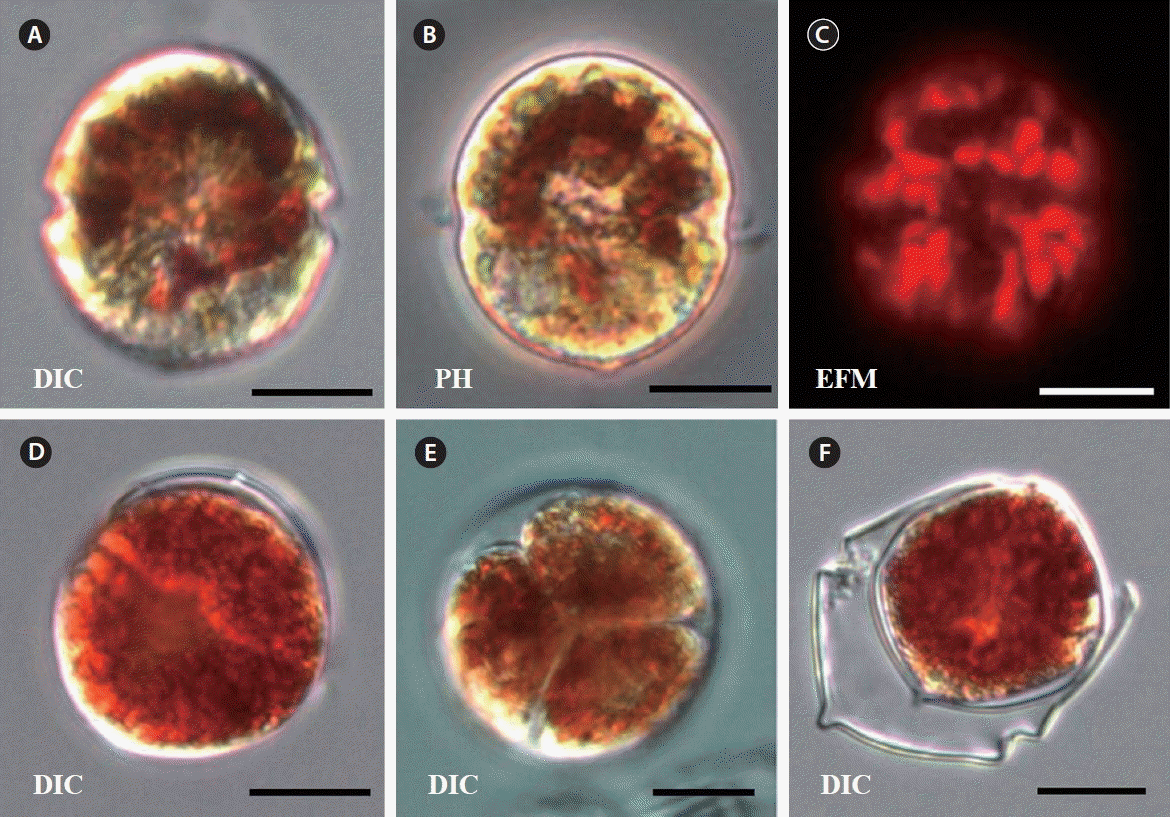
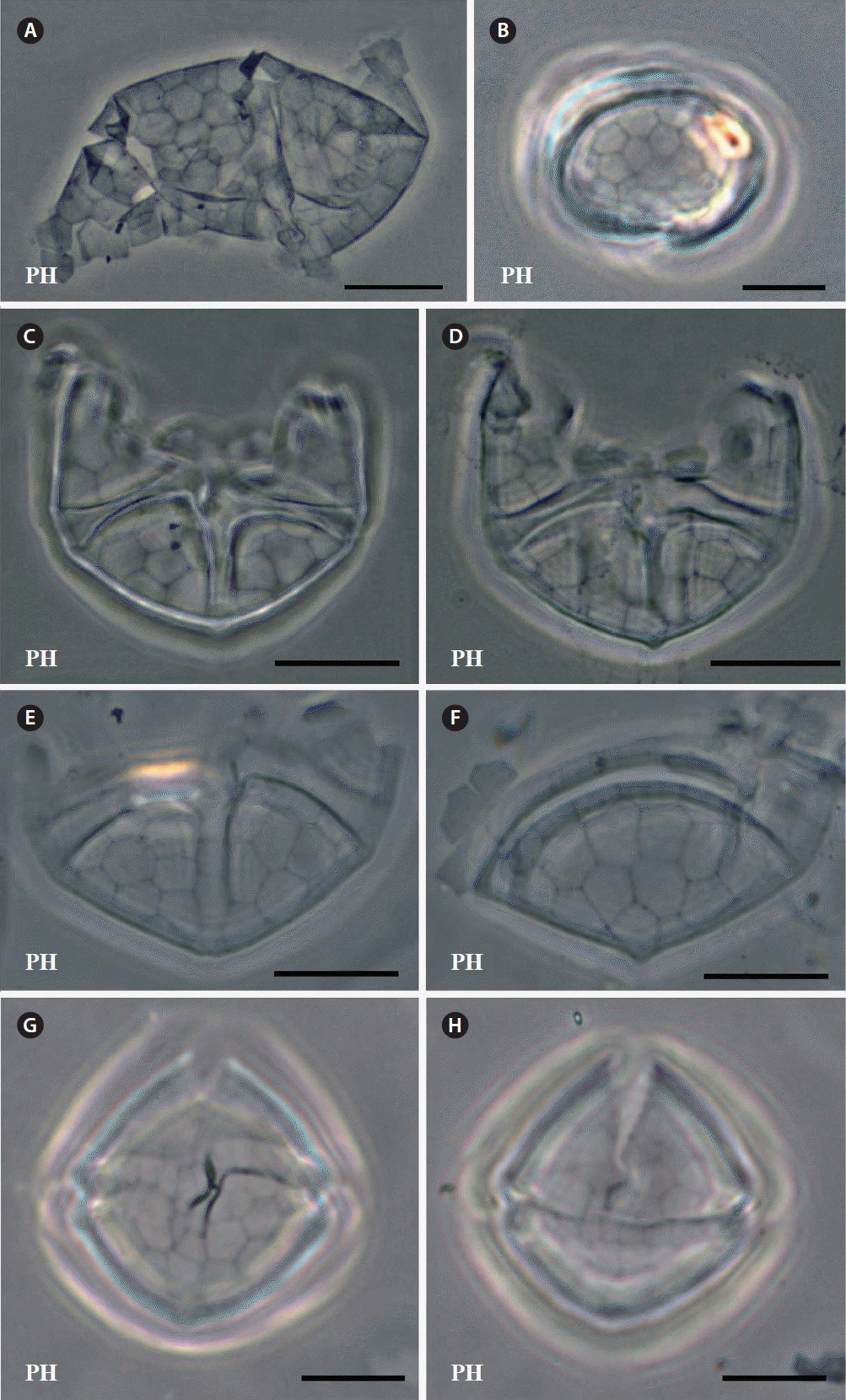
Berman-Frank, I, Zohary, T, Erez, J & Dubinsky, Z 1994. CO
2 availability, carbonic anhydrase, and the annual dinoflagellate bloom in Lake Kinneret. Limnol Oceanogr. 39:1822–1834.

Bolognini, N 1877. Salita alla Cima Roma (300 m circa) il 26 agosto 1875. Annuario SAT, Milano, 69–82.
Cantonati, M, Tardio, M, Tolotti, M & Corradini, F 2003. Blooms of the dinoflagellate
Glenodinium sanguineum obtained during enclosure experiments in Lake Tovel (N. Italy). J Limnol. 62:79–87.

Christen, HR 1958. Gymnodinium nygaardi sp. nov. Ber Schweiz Bot Ges. 68:44–49.
Corradini, F, Flaim, G & Pinamonti, V 2001. Five years of limnological observations on Lake Tovel 1995–1999 some considerations and comparisons with past data. Proc Ital Assoc Oceanol Limnol. 14:209–218.
Darriba, D, Taboada, GL, Doallo, R & Posada, D 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 9:772 pp.


Eker, E, Georgieva, L, Senichkina, L & Kideys, AE 1999. Phytoplankton distribution in the western and eastern Black Sea in spring and autumn 1995. ICES J Mar Sci. 56(Suppl):15–22.

Frassanito, R, Flaim, G, Mancini, I & Guella, G 2006. High production of unexpected carotenoids in Dinophyceae. Astaxanthin esters from the freshwater dinoflagellate
Tovellia sanguinea. Biochem Syst Ecol. 34:843–853.

Fukuju, S, Takahashi, T & Kawayoke, T 1998. Statistical analysis of freshwater red tide in Japanese reservoirs. Water Sci Technol. 37:203–210.

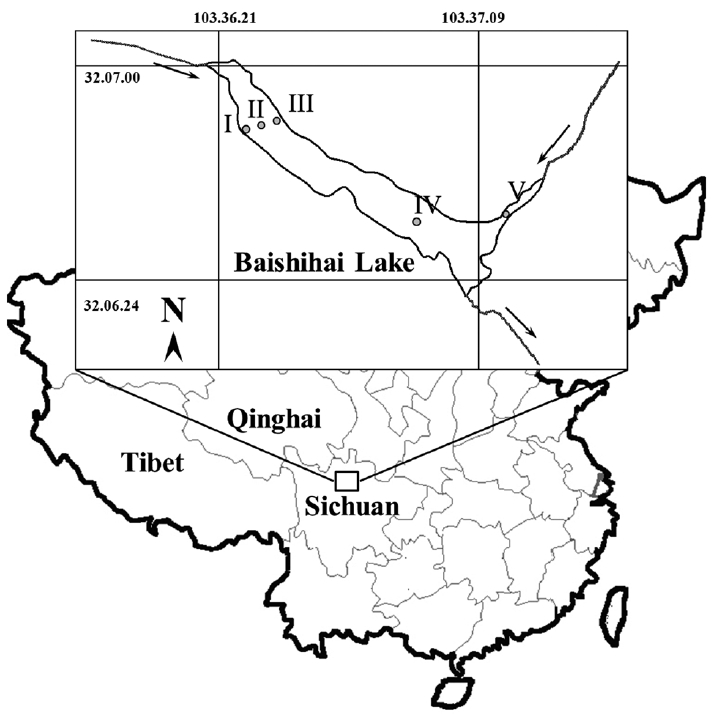
Jiang, H, Mao, X, Xu, H, Yang, H, Ma, X, Zhong, N & Li, Y 2014. Provenance and earthquake signature of the last deglacial Xinmocun lacustrine sediments at Diexi, East Tibet. Geomorphology. 204:518–531.

Hall, TA 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 41:95–98.
Huelsenbeck, JP & Ronquist, F 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755.


Ki, J-S, Jang, GY & Han, M-S 2005. Integrated method for single-cell DNA extraction, PCR amplification, and sequencing of ribosomal DNA from harmful dinoflagellates
Cochlodinium polykrikoides and
Alexandrium catenella. Mar Biotechnol. 6:587–593.

Li, Z, Shin, HH & Han, M-S 2015. Morphology and phylogeny of a new woloszynskioid dinoflagellate
Tovellia paldangensis sp. nov. (Dinophyceae). Phycologia. 54:67–77.

Lindberg, K, Moestrup, Ø & Daugbjerg, N 2005. Studies on woloszynskioid dinoflagellates. I:
Woloszynskia coronata re-examined using light and electron microscopy and partial LSU rDNA sequences, with description of
Tovellia gen. nov. and
Jadwigia gen. nov (Tovelliaceae fam. nov.). Phycologia. 44:416–440.

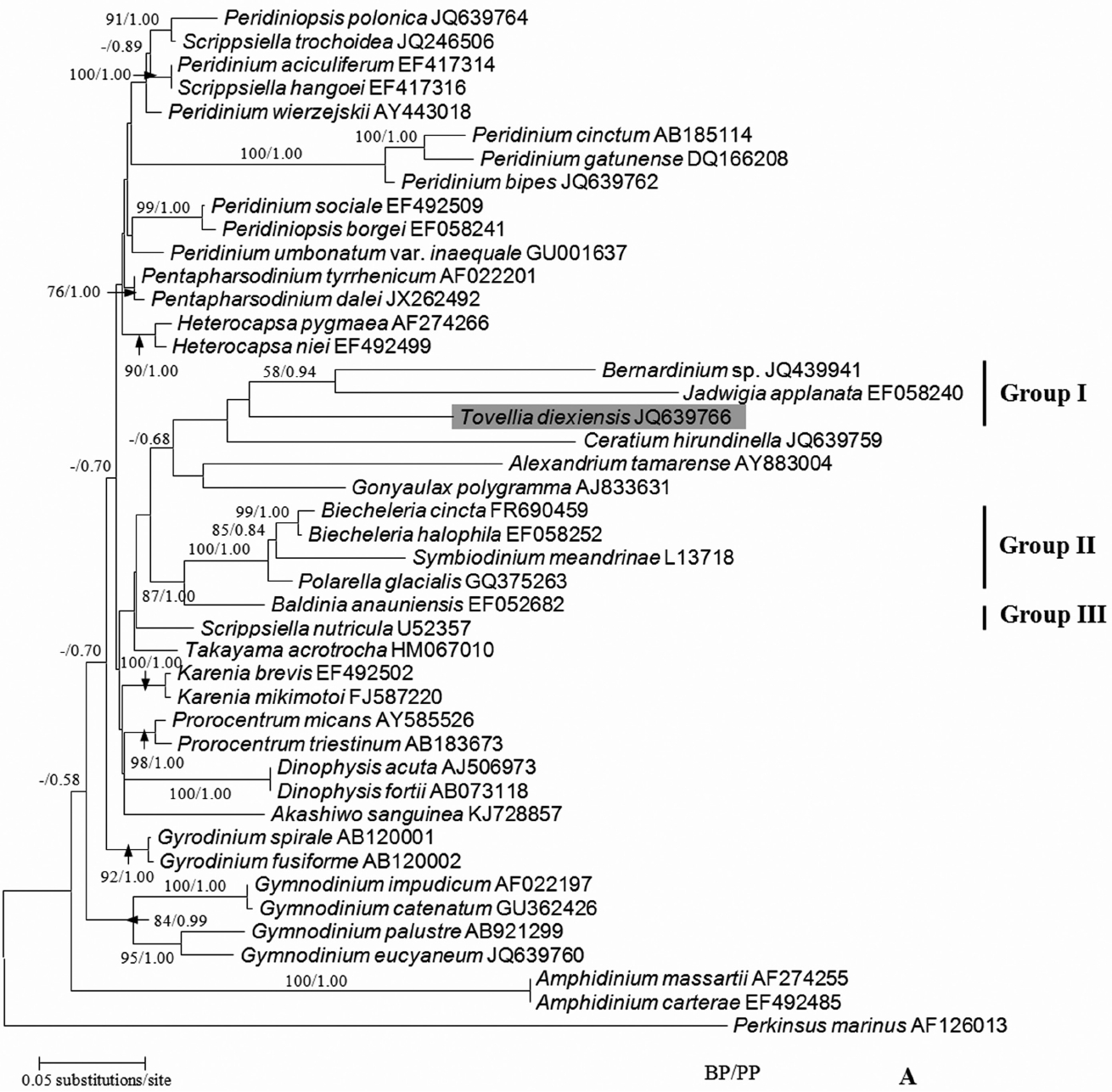
Logares, R, Shalchian-Tabrizi, K, Boltovskoy, A & Rengefors, K 2007. Extensive dinoflagellate phylogenies indicate infrequent marine–freshwater transitions. Mol Phylogenet Evol. 45:887–903.


Lorenzen, CJ 1967. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr. 12:343–346.

Lund, JWG, Kipling, C & Le Cren, ED 1958. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia. 11:143–170.

Marchesoni, V 1959. La Val di Tovel e il “Lago Rosso”. Nat Alp. 10:37–76.
Moestrup, Ø & Daugbjerg, N 2007. On dinoflagellate phylogeny and taxonomy. In : Brodie J, Lewis J, editors
Unravelling the Algae. CRC Press, FL, 215–230.

Moestrup, Ø, Hansen, G & Daugbjerg, N 2008. Studies on woloszynskioid dinoflagellates III: on the ultrastructure and phylogeny of
Borghiella dodgei gen. et sp. nov., a cold-water species from Lake Tovel, N. Italy, and on
B. tenuissima comb. nov. (syn.
Woloszynskia tenuissima). Phycologia. 47:54–78.

Moestrup, Ø, Hansen, G, Daugbjerg, N, Flaim, G & D’Andrea, M 2006. Studies on woloszynskioid dinoflagellates II: on
Tovellia sanguinea sp. nov., the dinoflagellate responsible for the reddening of Lake Tovel, N. Italy Eur J Phycol. 41:47–65.

Pandeirada, MS, Craveiro, SC, Daugbjerg, N, Moestrup, Ø & Calado, AJ 2014. Studies on woloszynskioid dinoflagellates VI: description of
Tovellia aveirensis sp. nov. (Dinophyceae), a new species of Tovelliaceae with spiny cysts. Eur J Phycol. 49:230–243.

Regel, RH, Brookes, JD & Ganf, GG 2004. Vertical migration, entrainment and photosynthesis of the freshwater dinoflagellate
Peridinium cinctum in a shallow urban lake. J Plankton Res. 26:143–157.

Rodriguez, S, Couté, A, Tenhage, L & Mascarell, G 1999. Peridiniopsis durandi sp. nova (Dinophyta), a new freshwater dinoflagellate causing red tides. Algol Stud. 130:15–29.
Schlüter, L & Havskum, H 1997. Phytoplankton pigments in relation to carbon content in phytoplankton communities. Mar Ecol Prog Ser. 155:55–65.

Shyam, R & Sarma, YSRK 1975.
Woloszynskia stoschii and
Gymnodinium indicum, two new freshwater dinoflagellates from India: morphology, reproduction and cytology. Plant Syst Evol. 124:205–212.

Stamatakis, A 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22:2688–2690.


State Environmental Protection Bureau (SEPB). 2002. Methods of monitoring and analysis for water and wastewater. 4th ed. China Environmental Science Press, Beijing, 200–285.
Tamura, K, Dudley, J, Nei, M & Kumar, S 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 24:1596–1599.


Thompson, RH 1951. A new genus and new records of fresh-water Pyrrophyta in the Desmokontae and Dinophyceae. Lloydia. 13:277–299.
Tomasi, G 1989. Dall’immaginario al plausibile. Nat Alp. 40:1–72.
von Stosch, HA 1973. Observations on vegetative reproduction and sexual life cycles of two freshwater dinoflagellates,
Gymnodinium pseudopalustre Schiller and
Woloszynskia apiculata sp. nov. Br Phycol J. 8:105–134.

Wang, XQ, Li, YR, Yuan, Y, Zhou, Z & Wang, LS 2014. Palaeoclimate and palaeoseismic events discovered in Diexi barrier lake on the Minjiang River, China. Nat Hazard Earth Sys. 14:2069–2078.

Wołoszyńska, J 1917. Neue Peridineen-arten, nebst Bemerkungen über den Bau der Hülle bei Gymnodinium und Glenodinium. Bull Int Acad Pol Sci Lett Ser B Sci Nat. 17:114–122.
Xu, X-N & Wang, L-S 2002. Mountain hazard caused by earthquake in Songping River upper Minjiang and its controlling. Chin J Geol Hazard Control. 13:31–35.
Yuan, J-P, Chen, F, Liu, X & Li, X-Z 2002. Carotenoid composition in the green microalga
Chlorococcum. Food Chem. 76:319–325.

Zhang, Q, Liu, G & Hu, Z 2011. Morphological differences and molecular phylogeny of freshwater blooming species,
Peridiniopsis spp. (Dinophyceae) from China. Eur J Protistol. 47:149–160.


Zhang, ZS & Huang, XF 1991. The research methods for freshwater plankton. Science Press, Beijing, 333–356.