Effects of salinity and irradiance on early developmental stages of Grateloupia turuturu (Halymeniaceae, Rhodophyta) tetrasporophytes
Article information
Abstract
Grateloupia turuturu is a red alga with a flat but firm slippery thallus. Throughout its lifetime, this alga experiences a wide range of environmental stresses in the intertidal rocky shores. The aim of this study is to investigate the effect of salinity and irradiance on the early developmental stages of G. turuturu tetrasporophytes. The released carpospores were cultivated at different salinities (S = 15, 25, and 35) and irradiances (50, 100, and 200 μmol photons m−2 s−1). Germination of carpospores and development of juvenile tetrasporophytes were observed every 5 days and recorded by a digital camera. Discoid crusts were formed at all conditions within 5 days. The discoid crusts at 200 μmol photons m−2 s−1 died within 20 days regardless the salinity. The discoid crusts at S = 35 also died at all irradiance conditions within 25 days. Except for those at S = 35 and 200 μmol photons m−2 s−1, the discoid crusts reached about 8,000–9,000 μm2 by day 20. Regardless of irradiance, the upright thalli formation rate from discoid crusts was 85 and 10% at S = 15 and S = 25, respectively. These results suggest that salinity and irradiance are important factors influencing early developmental stages of G. turuturu.
INTRODUCTION
Grateloupia turuturu Y. Yamada 1941 (Halymeniaceae, Rhodophyta) is a red macroalga native to East Asia (Denis et al. 2010, Capistrant-Fossa and Brawley 2019). It has a worldwide distribution and is considered an invasive species in many parts of the world (Villalard-Bohnsack and Harlin 1997, 2001, Saunders and Withall 2006, D’Archino et al. 2007, de Azevedo et al. 2015, Bolton et al. 2016, Kraemer et al. 2017, Petrocelli et al. 2020). This alga has slippery blades that range from a dark red to pink to red-tinted brown, with extremely variable morphologies occurring in intertidal and subtidal environments (Simon et al. 1999, 2001, Lafontaine et al. 2011). They have a discoid holdfast (<5 mm wide), with a short (<1.5 mm long) cylindrical stipe, which widens out into a flattened blade (Villalard-Bohnsack and Harlin 1997).
In Asia, G. turuturu is considered to be an economically important species. It is rich in dietary fiber, protein, and vitamins (Lordan et al. 2011). Additionally, some chemical compounds from G. turuturu can be used for antibacterial and anti-microfouling applications (Plouguerné et al. 2008, Cardoso et al. 2019).
Grateloupia turuturu grows slowly during the winter with little changes in length, standing crop, and density in Korea. It has an isomorphic haplo-diplontic life cycle (Cardoso et al. 2019). It is a prolific spore producer from either the gametophyte or tetrasporophyte stages. The sporelings develop into perennating crusts, from which blades can be repeatedly produced (Harlin and Villalard-Bohnsack 2001, Shao et al. 2004). Standing crop biomass and density increases during the spring (Araújo et al. 2011). After the carpospores are released, discoid crusts are formed by irregular division of germinated carpospores (Wang et al. 2012).
Temperature, salinity, and irradiance have significant effects on the development of discoid crusts and the growth of upright thalli in Grateloupia qingdaoensis (Liu et al. 2020). Grateloupia turuturu survives well in temperatures from 4 to 28°C, and in salinities ranging from 15–37 (Simon et al. 2001). Carpospores are widely used for seedlings in its aquaculture because they are released in abundance from cystocarps (Glenn et al. 1996, Mantri et al. 2009). Moreover, the specific growth rate of crusts and thalli developed from carpospores was higher than that of the crusts developed from tetraspores of G. subpectinata (Adharini and Kim 2016). However, the effect of environmental conditions, including salinity and irradiance, on early developmental stages from G. turuturu carpospores is still relatively unknown (Wei et al. 2013). The aim of this study was to investigate the effects of salinity and irradiance on the germination of carpospores and the early developmental stages of tetrasporophyte in G. turuturu.
MATERIALS AND METHODS
Carpospores collection
The cystocarpic thalli of G. turuturu were collected from the intertidal rocky shores of Samcheok, Korea (36°26′ N, 129°11′ E). The salinity range at the collection site was from 22 to 26. The thalli were rinsed in filtered seawater and wiped with paper towels to remove epiphytes. The cystocarp thalli were excised by sterilized scalpel blades and then placed in between damp paper towels in the dark, overnight. To induce spore liberation, cystocarp thalli were placed in 250 mL Erlenmeyer flasks containing filtered and sterilized seawater with pH 7.5 and standard salinity condition (S = 35). The carpospores were released in 2 days (Wang et al. 2012) and transferred to Petri dishes (150 mm × 15 mm) containing five slide glasses (25 mm × 25 mm) and von Stosch’s enriched (VSE) medium (Ott 1965) with pH 7.5 and standard salinity condition (S = 35). These dishes were then placed at 20°C with 50 μmol photons m−2 s−1 irradiance and 12 : 12 h (L : D) photoperiod. After the settlement of carpospores on the glass slides, the slides were rinsed with filtered sterilized seawater to remove non-settled carpospores. The density of spores was about 16–18 spores per glass slide (2–3 spores cm−2). Five glass slides were transferred to new Petri dishes with VSE medium (Wang et al. 2012).
Experimental treatments
Nine treatment combinations of salinity (S = 15, 25, and 35) and irradiance (50, 100, and 200 μmol photons m−2 s−1) were used. The experiment was performed at 20°C with a photoperiod of 12 : 12 h, L : D for 25 days. The VSE medium was renewed every 5 days. Each replicate was composed of one Petri dish with five slide glasses, each treatment combination had three replicates.
Observations of early developmental stages
Development of tetrasporophyte germlings was observed every 5 days using a Nikon Ts2R microscope (Tokyo, Japan). Thirty discoid crusts were randomly selected from each Petri dish every 5 days and photographed with a KOPTIC HK6.3E3S digital camera (Yongin, Korea). The formation of discoid crusts and upright thalli were recorded, and the measurement of discoid crust area with highly pigmented regions was conducted using Image-J software (version 1.52a, https://imagej.nih.gov/ij/).
Statistical analysis
Two-way ANOVA and Tukey’s honest significance test (p < 0.05) were conducted for the discoid crust area to test differences among treatment combinations. All data were checked for normality using Kolmogorov-Smirnov test and homogeneity of variance using Levene’s test. The statistical analysis was performed on the statistical software SPSS version 25.0 (IBM Corp., Armonk, NY, USA).
RESULTS AND DISCUSSION
Formation of discoid crusts
The carpospores released from cystocarps were settled on the slide glasses within 2 days. After settlement, carpospores began to germinate and form discoid crusts at all treatment combinations within 5 days (Fig. 1). Similarly, Wei et al. (2013) reported that irradiance had no significant effect on the formation of discoid crusts from filaments of G. turuturu (Wei et al. 2013). Carpospores of G. qingdaoensis germinated and grew into discoid crusts at the photosynthetically active radiation (PAR) ranged from 20 to 120 μmol photons m−2 s−1 and salinity ranged from S = 20 to S = 40 (Adharini and Kim 2016). In the present study, similarly, carpospores of G. turuturu germinated at higher PAR (200 μmol photons m−2 s−1) and lower salinity (S = 15). The area of discoid crusts was not affected by salinity (p > 0.05), irradiance (p > 0.05), and interaction between salinity and irradiance (p > 0.05) with the area of approximately 760 μm2 (Figs 1 & 2A).

Discoid crusts of Grateloupia turuturu for each treatment combination at day 5. Scale bar represents: 50 μm.
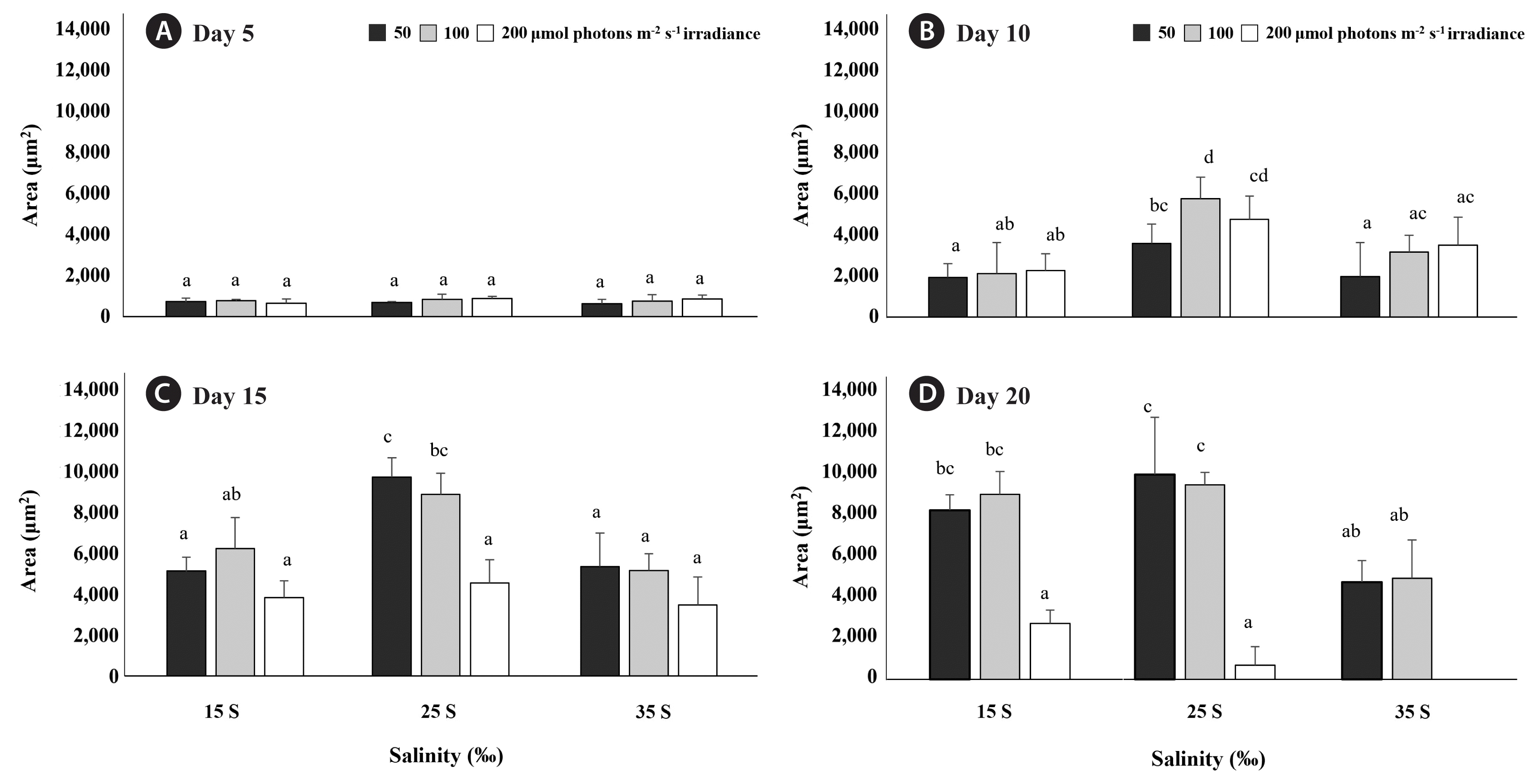
Randomly selected 30 Grateloupia turuturu discoid crust areas of each treatment combinations salinity (S = 15, 25, and 35) and irradiance (50, 100, and 200 μmol photons m−2 s−1) at day 5 (A), day 10 (B), day 15 (C), and day 20 (D). Values are means ± standard errors. Different letters represented significant difference of each day (p < 0.05).
Effects of salinity and irradiance on the growth of the discoid crust
At day 10, the growth of discoid crusts was significantly affected by salinity (p < 0.001), irradiance (p < 0.001), and interaction between salinity and irradiance (p = 0.047). The area of discoid crusts was increased in all treatment combinations and greater at S = 25 and 100 μmol photons m−2 s−1 than at all other treatment combinations (p < 0.001) (Fig. 2B). At day 15 and 20, the growth of discoid crusts was significantly affected by salinity (p < 0.001), irradiance (p < 0.001), while interaction between salinity and irradiance did not affect the growth of discoid crusts (p > 0.05). At day 15, the area of discoid crusts at 50 and 100 μmol photons m−2 s−1 reached about 9,000 μm2 at S = 25. However, at the same salinity, the area of discoid crusts at 200 μmol photons m−2 s−1 was significantly less than 9,000 μm2 (p < 0.001) (Fig. 2C). Additionally, discoid crusts at 200 μmol photons m−2 s−1 showed pigment degradation regardless the salinity. At day 20, the area of discoid crusts at 50 and 100 μmol photons m−2 s−1 also reached about 8,000 μm2 at S = 15. Furthermore, the area of discoid crusts stopped increasing at 50 and 100 μmol photons m−2 s−1 at S = 25 after day 15. However, discoid crusts at S = 35 started to die at all irradiances by day 20 (p < 0.001) (Fig. 2D). Especially, the combination of S = 35 and 200 μmol photons m−2 s−1 hastened the death of the discoid crusts compared to other conditions (Fig. 3). Environmental factors of original habitat, especially salinity, affected the early developmental stage of G. turuturu. Salinity of 35 is normal in oceanic environments, but discoid crusts of G. turuturu at this salinity started to die at all irradiances at day 20 in the present study, interestingly, S = 25 enhanced the growth of discoid crust compared to salinity of 35 under 50 and 100 μmol photons m−2 s−1 from day 10 to day 15. The salinity of 25 corresponds with the salinity at the collection site. This result implies the adaptation of this alga to the local environment, where freshwater influxes occur. Similar phenomenon was also found in Caloglossa leprieurii and Bostrychia radicans (Yarish et al. 1979, 1980), and in Gracilaria (Yu et al. 2013). Genetic structure differences were observed between estuarine populations of Caloglossa and Bostrychia (Yarish et al. 1979) and for Furcellaria lumbricalis from Atlantic Ocean (S = 35) and brackish Baltic Sea (S = 3.6), suggesting salinity of original habitat affected physiological responses through the differences of genetic structure (Korpelainen 2016). Additionally, the tolerance to temperature and salinity varies between sporelings and mature thalli of Grateloupia doryphora (sensu lato G. turuturu, Rodrigo and Robaina 1997), suggesting the life stage also affects stress tolerance.

Grateloupia turuturu discoid crusts of each treatment combinations at day 20. Scale bar represents: 50 μm.
Irradiance also affects the growth of discoid crusts. Except for discoid crusts at S = 25 and 100 μmol photons m−2 s−1 at day 10, there was no differences of discoid crust areas between 50 and 100 μmol photons m−2 s−1 at S = 15, 25, and 35. However, at 200 μmol photons m−2 s−1 caused growth inhibition with pigment degradation occurred. In previous studies, discoid crusts grown from carpospores of Grateloupia constricata and G. qingdaoensis had slow growth under high irradiances (Ding et al. 2020, Liu et al. 2020). Similarly, excessive irradiance caused pigment degradation of discoid crusts of Grateloupia asiatica (Adharini and Kim 2014).
Formation of upright thalli
Iima et al. (1995) reported that upright thalli of Grateloupia acuminata were formed when the discoid crust reached a certain size. In case of Grateloupia asiatica, upright thalli were formed when discoid crust were composed of about six layers of cells (Adharini and Kim 2014). According to our observations, discoid crusts with the area of approximately 8,000–9,000 μm2 started to form upright thalli at day 25 (Fig. 4). Wei et al. (2013) found that discoid crusts of G. turuturu with 200–300 μm (30,000–70,000 μm2) in diameter developed upright thalli. It is unclear why these size differences of discoid crusts were observed in different studies, and thus, further studies are needed to confirm the size required to develop upright thalli in G. turuturu.
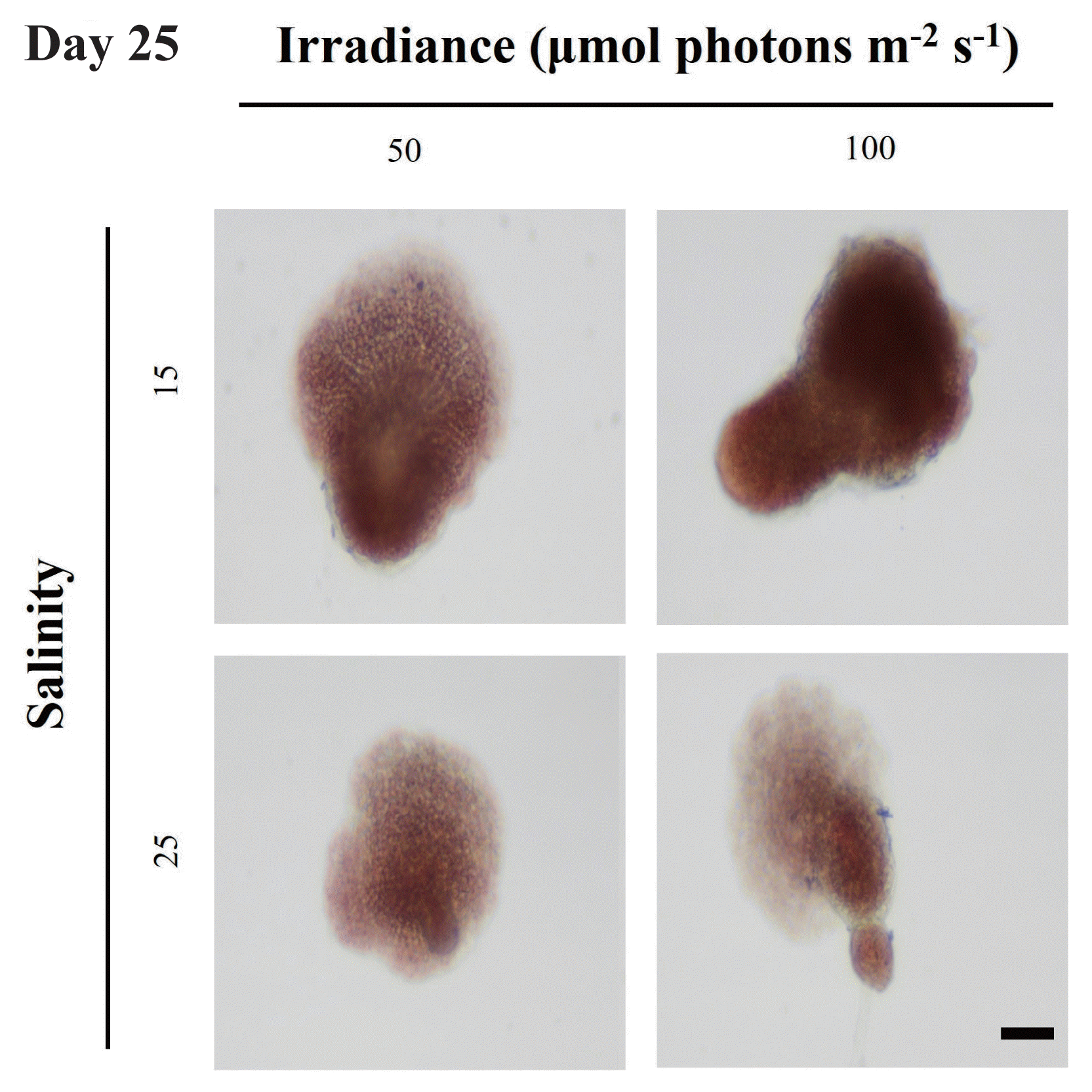
Upright thalli formation of Grateloupia turuturu in different treatments at day 25. Scale bar represents: 50 μm.
The formation rate of upright thalli from discoid crusts was 85 and 10% at S = 15 and S = 25, respectively, regardless of irradiance. Wei et al. (2013) reported that the upright thalli formation time of G. turuturu was faster at 90 μmol photons m−2 s−1 than those at lower irradiance under the same temperature. In the present study, irradiance did not affect the formation time of upright thalli. Although the growth of discoid crust at S = 25 was faster than at S = 15, the formation rate of upright thalli was significantly higher at S = 15 than S = 25. This result suggests that the main factor affecting the formation of upright thalli is salinity. However, the present study did not measure the growth of the upright thalli.
CONCLUSION
This is the first study looking at the combined effects of salinity and irradiance, on early development stages G. turuturu. The combination of high salinity (S = 35) and high irradiance (200 μmol photons m−2 s−1) inhibited the growth of discoid crusts. The minimum area of 8,000 μm2 was required to develop upright thalli. The upright thalli formation was only affected by salinity. The present study also suggests that the optimal salinity conditions for the early development of G. turuturu may be site dependent, but this needs to be confirmed using different populations from different salinity conditions.
ACKNOWLEDGEMENTS
This study was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1A6A1A06015181 and NRF-2022R1I1A1A01071940), and by the Ministry of Science and ICT (2022R1A2C1011394).
Notes
The authors declare that they have no potential conflicts of interest.