Polyphenol-rich Sargassum horneri alleviates atopic dermatitis-like skin lesions in NC/Nga mice by suppressing Th2-mediated cytokine IL-13
Article information
Abstract
Atopic dermatitis (AD) is one of major skin inflammatory diseases characterized by excessive Th2-mediated immune responses. Recent evidence provides that interlukin-13 (IL-13) plays the role of a key Th2 cytokine that drives the inflammation underlining AD. Due to adverse effects of commercially available synthetic drugs, the need for treatments based on natural products is gaining much attention. Sargassum horneri is an edible brown algae known for beneficial bioactivities including anti-inflammation. We investigated if polyphenol-rich S. horneri extracts (SHE) could suppress AD-like skin lesions in NC/Nga mice and if that involved inhibition of the infiltration of Th2-mediated cytokine IL-13. We observed markedly increased infiltration of IL-13 positive cells in AD-like skin lesions of mice but SHE treatments decreased it. Also, the dermal expression of IL-13 was sufficient to cause inflammatory responses in mice skin resembling human AD. SHE suppressed the dermal infiltration of inflammatory cells where IL-13 plays a crucial role in skin tissues and in the recruitment of inflammatory cells. Furthermore, it was confirmed that SHE reduced T cell, dendritic cell, and macrophage populations in spleen. Moreover, SHE decreased the collagen deposition in skin and ear dermis resulting in reduced fibrosis that occurs in AD due to excessive collagen. Taken together, our results reveal that SHE suppressed the infiltration of inflammatory cells into skin dermis by decreasing the infiltration of IL-13 positive cells. Therefore, SHE could be taken as a useful therapeutic agent to alleviate AD.
INTRODUCTION
Atopic dermatitis (AD) is the most common chronic skin inflammatory disease characterized by itchy and relapsing inflammatory condition resulting in itchy, swollen, and cracked skin (Bieber 2010). It is known to affect 15–20% of children and 1–3% of adults (Zheng et al. 2009), and its incidence has steadily increased in past decades (Leung et al. 2004).
Since skin inflammation results from inflammatory cells’ response to cytokines, they are critical factors in the pathogenesis of AD (Nomura et al. 2003). It is well accepted that deviated Th2 immune responses are closely linked to AD, and major Th2-mediated cytokines interleukin (IL)-4, IL-5, and IL-13 are detected in both lesion and non-lesion stages of skin during the acute phase of AD (Bieber 2010). In particular, IL-13 provides a strong evidence of a dominant cytokine in the inflammatory condition of AD (Bieber 2020). In dermal compartment of skin, macrophages and dendritic cells (DCs) have been considered as the main resident immune cells, and besides lymphocytes other than the mast cells, basophils are considered as a source of key cytokines like IL-13 in inflammatory responses (Bieber 2020). Moreover, infiltration of eosinophils forms the pivotal character in AD since the recruitment and the function of eosinophils are mediated by Th2 cytokines (Kim et al. 2016). The Th2-related cytokines are overexpressed in acute AD lesions (Gittler et al. 2012), and cytokines, particularly IL-13, stimulate keratinocytes to attract CD4+ T cells by way of chemokine production (Bieber 2020). Furthermore, IL-13 demonstrated to induce significantly increased collagen deposition and fibrosis in skin (Zheng et al. 2009).
Treating AD usually involves the use of corticosteroids, but extended use is usually not recommended since it can increase the risk of toxicities, skin atrophy, adrenal suppression and growth retardation (Simpson et al. 2017). Therefore, strong attention has been raised for new anti-inflammatory agents from natural products. In particular, marine organisms could be considered as a treasure trove since they are known to offer materials of diverse structures and various biological activities (Jeong et al. 2014).
Sargassum horneri is an edible brown seaweed and mostly distributes along coasts of Korea, Japan, and China (Sanjeewa et al. 2019, Hwang and Park 2020). It has been considered as a source of food and drug in traditional Chinese medicine (Zheng et al. 2009). It was found that S. horneri consists of a great amount of nutrition like dietary fibers, vitamins, amino acids, and polysaccharides (Kim et al. 2018). Polyphenols and polysaccharides that have beneficial functions are also common in S. horneri (Herath et al. 2019). For instance, polyphenols have been considered as an active component in brown macroalgae including Sargassum sp. (Generalić Mekinić et al. 2019), including anti-inflammatory effect (Namvar et al. 2013). For our current study, we used a S. horneri extract (SHE) that is rich with polyphenols and additionally with calorie (228.24 kcal 100 g−1), protein (16.84% dry weight [DW] basis), ash (28.4% DW basis), and carbohydrates (39.9% DW basis) to investigate its potential to treat AD (Herath et al. 2019). Since polyphenols have strong anti-inflammatory potential, we already found beneficial effects of SHE against 2,4-dinitrochlorobenzene (DNCB)-induced Th2-mediated inflammatory responses in AD. Notably, we found that SHE could counter the abnormal immune responses in house dust mites (HDM) / DNCB-induced AD mouse models (Han et al. 2020). Moreover, SHE has suppressive effects on concanavalin A induced activation of immune cells such as granulocytes, eosinophils, T cells, and monocytes in murine splenocytes (Herath et al. 2019).
Previously, we showed that sargachromenol, a chrome compound from SHE, was able to protect skin damages induced by ultraviolet A through inhibiting the AP-1 transcription (Kim et al. 2012). Also recent in vitro and in vivo studies revealed that brown seaweeds have great therapeutic potential against inflammatory reactions (Wijesinghe and Jeon 2011). Interestingly, despite known importance of inflammation, little studies examined seaweeds’ efficacy to alleviate AD symptoms (Han et al. 2020). We hypothesized that SHE would improve AD via anti-inflammatory activity and investigated its preventive therapeutic effect against DNCB-induced inflammatory responses in an AD mouse model. In this study, we examined the infiltration of inflammatory cells in AD skin focusing on Th2-mediated cytokine IL-13, inflammatory mediators, and collagen deposition in DNCB-induced AD mice. This study provides an evidence that SHE can alleviate AD-like skin lesions through inhibiting Th2-mediated inflammatory responses.
MATERIALS AND METHODS
Preparation of seaweed extract
S. horneri was obtained along the coasts of Jeju Island in Korea, and seaweed extracts (lot number SJFC70180625, 201013) were prepared. The 70% ethanol extract of S. horneri (SHE) was prepared as described previously (Herath et al. 2019, Kim et al. 2020). In brief, the S. horneri samples were washed in pure water and dried. The dried samples made into fine particles by passing through a 40–50 mesh using a Pin-mill and dissolved in 70% ethanol. It was centrifuged (12,000 rpm), treated with 95% ethanol, and concentrated. The prepared samples were stored -20°C.
Animals
Eight-week-old female NC/Nga mice were purchased from Orient Bio (Gwangju, Korea) and housed in individual ventilated cages under specific pathogen-free conditions at constant temperature 23 ± 1.5°C and 55 ± 15% humidity with a 12 h light-dark cycle. Mice were reared with conventional facilities and approved diet and water ad libitum. All experimental protocols were approved by the Institutional Animal Care and Use Committee of Chonnam National University (No. CNU IACUC-YS-2016-6).
Induction of experimental AD
To induce experimental AD skin lesions, we shaved dorsal hairs of NC/Nga mice with an electronic shaver. A hair removal cream and sodium dodecyl sulfate were applied to the shaved areas 3 h before the application of HDM / DNCB and AD cream (Biostir-AD; Biostir, Kobe, Japan). The induction of AD to dorsal skin of mice was indicated in Supplementary Fig. S1. Mice were randomized into five groups at the 14th day of AD induction as naïve (control, n = 8), DNCB group (HDM / DNCB applied mice, n = 8), DNCB + SHE10 group (HDM / DNCB and SHE 10 mg kg−1 co-applied mice, n = 8), DNCB + SHE100 groups (HDM / DNCB and SHE 100 mg kg−1 co-applied mice, n = 8), and DNCB + CJLP133 group (a positive control group with HDM / DNCB and CJLP133 800 mg kg−1 co-applied mice, n = 8). The Lactobacillus plantarum CJLP133 isolated from Kimchi was found to suppress AD-like skin lesions and diminished the sizes of topical inflammatory sites in NC/Nga mice (Han et al. 2012).
Differential cell count in blood
Mice were euthanized, and peripheral blood was collected through cardiac puncture using heparinized syringes to an ethylene diamine tetra acetic acid tube. Smears were made and air-dried for Diff Quick stain for differential leukocyte counting. Cell differentiation was determined by counting 100 cells, and cells were classified as lymphocytes, neutrophils, monocytes, eosinophils, and basophils using a microscope camera (Olympus, Tokyo, Japan) system.
Histopathological analysis of skin and ear
The skin and ear tissues were fixed in a 10% formalin and embedded in paraffin. Sections of 3 μm widths were cut and stained with hematoxylin and eosin (H&E) stain to assess histopathological changes. Congo red staining was performed to identify eosinophils. Masson’s trichrome staining was performed to analyze collagen fibers in skin and ear tissues using standard procedures. Sections were dehydrated in a series of ethanol and cleared in xylene. Each of sections was sealed with Canada balsam and monitored using an Olympus DP-72 microscope camera (Olympus).
Immunohistochemical analysis of skin and ear
Immunohistochemistry (IHC) was performed on formalin–fixed, paraffin–embedded skin tissue sections by using horseradish peroxidase-labeled Vectastain Elite ABC kits (Vector Laboratories, Peterborough, UK) according to manufacturer’s instructions. Sections were deparaffinized and rehydrated through a graded series of ethanol. Endogenous peroxidase was blocked by incubating in 0.3% hydrogen peroxide for 30 min followed by an incubation with a blocking serum (Vector Laboratories) to block nonspecific binding. Sections were incubated overnight at 4°C with antibodies against CD4 (1 : 200; Novus Biologicals, Centennial, CO, USA), anti-mouse CD45R/B220 (1 : 200; BD Pharmingen, San Diego, CA, USA), anti-F4/80 (1 : 200; BioLegend, San Diego, CA, USA), CD11c (1 : 200; AbD seroTec, Kidlington, UK), IL-13 (1 : 200; Santa Cruz Biology, Santa Cruz, CA, USA), anti-inducible nitric oxide synthase (iNOS) (1 : 200; Millipore, Burlington, MA, USA), and cyclooxygenase-2 (COX-2; 1 : 400; Santa Cruz Biology). After washing, tissues were incubated with biotinylated secondary antibodies for 45 min. Phosphate buffer solution (PBS) was used to wash tissues and subsequently a solution containing avidine : biotine peroxidase complex was applied to sections for 45 min. Diaminobenzidine (Vector Laboratories) was used to visualize the immunoreactivity and counterstained with hematoxylin (Dako, Carpinteria, CA, USA). Images were collected by using a microscope (Olympus), and positive cells were quantified using a computer-assisted software (ImageJ v1.46; National Institute of Health, Bethesda, MD, USA).
Flow cytometry analysis
Splenocytes were excised, and single cell suspensions were prepared in RPMI-1640 medium. Cells were washed in PBS and centrifuged at 1,500 rpm for 5–6 min. Single cell suspensions (1 × 106) were incubated with an Fc receptor-blocking antibody (BD Bioscience, San Jose, CA, USA) at 4°C. Then cells were stained with fluorophore antibodies CD4 (H129), CD11b (M1/70), CD11c (HL3) purchased from BioLegend and BD Biosciences (Franklin Lakes, NJ, USA) with one of following tags, phycoerythrin, peridinin chlorophyll protein-Cy5.5, allophycocyanin. Finally, cells of each sample were analyzed by a CytoFLEx flow cytometer (Bio-Health Materials Core-Facility Centre, Jeju National University) and CytExpert 1.2 software (Backman Coulter, Inc., Brea, CA, USA). The CD4+ gate represents the set of lymphocytes according to forward and side scatter properties while CD11b+ and CD11c+ were gated out from the whole acquired events by a combination of physical single gates (forward scatter vs. slide scatter) with vital CD45+ gate. All cell populations were displayed as a histogram and printed out for each experiment.
Statistical analysis
Results of each experiment were expressed as mean ± standard deviation. Student t-test was performed using Microsoft Office Excel 2013 program (Redmond, WA, USA), and p < 0.05 was determined significant.
RESULTS
SHE mitigated DNCB-induced inflammatory cell count in blood
Differential count of inflammatory cells in blood was done to evaluate the effect of SHE on inflammation. We observed significant increase of granulocytes by 2.5 folds in DNCB group while SHE treatments did not reduce it significantly compared to DNCB group (Fig. 1A). Monocyte (Fig. 1B) and lymphocyte (Fig. 1C) cell counts did not show significant difference from that in DNCB group either. And neutrophils, basophils, and eosinophils counts did not change significantly in AD mice model (Fig. 1D–F). However, based on results it could be suggested that elevated granulocytes percentage could be exhibits in vehicle group. This data indicates that SHE suppresses the inflammatory cells in blood performing the anti-inflammatory effect.

Differential cell counts in blood. Percentages of granulocyte (A), monocyte (B), lymphocyte (C), neutrophil (D), eosinophil (E), and basophil (F) in blood. DNCB, 2,4-dinitrochlorobenzene; SHE, Sargassum horneri ethanol extracts. Data are represented as the mean ± standard error. *p < 0.05 indicates statistically significant increase compared to naïve group.
SHE suppressed infiltration of inflammatory cells in DNCB-induced AD mice skin and ear
Skin and ear sections were stained with H&E staining in order to evaluate anti-inflammatory effect of SHE on AD mouse model. Infiltration of inflammatory cells was significantly increased in DNCB group compared to naïve group in skin (Fig. 2B). However, DNCB + SHE10, DNCB + SHE100, and DNCB + CLJP133 showed relatively lower inflammatory scores compared to DNCB group. As shown in Fig. 2C, severe infiltration of inflammatory cells could be observed in DNCB group relative to that in naïve group (p < 0.0005). As expected, SHE treatment reduced the inflammatory score compared to DNCB group in a dose-dependent manner. Histopathological changes reflected similar trend: DNCB aggravated inflammatory lesions in both skin and ear of AD mice while SHE gradually improved them in a similar manner. These results signify that SHE was effective in decreasing inflammatory cells in both skin and ear tissues of DNCB-induced AD mice.

Effect of Sargassum horneri ethanol extracts (SHE) on 2,4-dinitrochlorobenzene (DNCB)-induced histological changes in skin and ear of DNCB-induced atopic dermatitis mice. Representative hematoxylin and eosin staining images of skin (I–V) and ear (VI–X) sections (A). Inflammatory cell infiltrations are indicated by arrowheads. The inflammation scores of mice skin (B) and ear (C) were assigned on a subjective scale score (I, none; II, mild to moderate; III, severe) and were separately analyzed. ***p < 0.005 indicates significant increase compared to naïve group. †p < 0.05, ††p < 0.01, and †††p < 0.005 indicate significant decrease compared to DNCB group. Scale bars represent: please change to 25 μm.
SHE reduced the infiltration of iNOS and COX-2 positive cells in dermis of skin and ear of DNCB-induced AD mice
To determine whether SHE treatments regulate inflammatory responses, we performed IHC analysis of iNOS positive cells in skin and ear. In inflammation, iNOS is produced through the production of nitric oxides and the occurrence of iNOS positive reactions in dermal macrophages. Accordingly, a significantly increased number of iNOS positive cells were observed (by 2.6 folds, p < 0.0005) in DNCB group compared to that in naïve group in skin tissues. The DNCB + SHE10 and DNCB + SHE100 groups showed decreased numbers of iNOS positive cells compared to that in DNCB group though not statistically significant (Fig. 3C). In ear tissues, a large number of iNOS positive cells were observed in DNCB group while a decreased number of iNOS positive cells were observed in DNCB + SHE10 and DNCB + SHE100 groups. DNCB + CJLP133 group showed a significantly decreased number of iNOS positive cells compared to that in DNCB group (by 2.3 folds, p < 0.05) (Fig. 3E). These results suggest that SHE attenuated the iNOS positive cells in skin and ear dermis of DNCB-induced AD mice.
Sargassum horneri ethanol extracts (SHE) reduced inflammation mediators inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice skin and ear. Representative images of iNOS (A) and COX-2 (B) positive cells (arrowheads) in skin (I–V) and ear (VI–X) sections are shown. The number of iNOS and COX-2 positive cells per site in skin (C & D) and ear (E & F) are analyzed. Data are presented as the mean ± standard error. ***p < 0.0005 indicates significant increase compared to naïve group. †p < 0.05 and †††p < 0.0005 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
We also measured COX-2 positive cells in skin and ear tissues of NC/Nga mice. In skin tissues, an increased number of COX-2 positive cells were detected in DNCB group (by 1.3 folds) compared to that in naïve group. And the number was decreased by SHE treatments though not significant (Fig. 3D). The DNCB group also represented a high number of COX-2 positive cells while it was decreased by SHE treatments in ear tissues though no significant changes were seen among different groups (Fig. 3F). Although the differences were not significant, it was consistently observed that SHE suppressed the number of COX-2 positive cells in both skin and ear tissues.
SHE suppressed eosinophil infiltration in DNCB-induced AD mice skin and ear
We further investigated the eosinophil infiltration in AD mice skin and ear with Congo red staining since the proliferation, migration, and local activation of eosinophils are characteristic features of AD. In mice skin, eosinophil count was found to be severely increased in DNCB group compared to that in naïve group, but SHE and CLJP133 treatments (DNCB + SHE10, DNCB + SHE100, and DNCB + CLJP133) dose dependently decreased the number of eosinophils compared to that in DNCB group (by 1.6, 1.8, and 1.9 folds, respectively; all p < 0.05) (Fig. 4B).

Congo red staining of skin and ear of 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Representative photographs of skin (I–V) and ear (VI–X) sections (arrowheads) stained with Congo red are shown (A). The number of Congo red stained cells per site in skin (B) and ear (C) are analyzed. SHE, Sargassum horneri ethanol extracts. Data are presented as the mean ± standard error. ***p < 0.0005 indicates significant increase compared to naïve group. †p < 0.05 and ††p < 0.005 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
Similarly, DNCB group ears of AD mice exhibited the highest eosinophil infiltration compared to that of naïve group, and the number of eosinophils recruited to the dermis was reduced in DNCB + SHE10 and DNCB + SHE100 groups compared to DNCB group by 2.3 (p < 0.05) and 1.9 (p < 0.005) folds, respectively. Also, DNCB + CJLP133 group suppressed the eosinophil infiltration compared to DNCB group (by 2.2 folds, p < 0.005) (Fig. 4C). These results indicate that SHE suppressed eosinophil infiltration in DNCB-induced AD mice. Overall, data indicate that SHE plays an important role in recruiting immune cells to skin and ear tissues of DNCB-induced AD NC/Nga mice.
SHE attenuated inflammatory infiltration of CD11c+ cells in skin and ear of DNCB-induced AD mice
DCs play a major role in antigen uptake and presentation, and induction of Th2-predominant responses. These DCs are easily stimulated by allergens while inflammatory subtype DCs are increased in AD skin (Kim et al. 2016). In present study, we determined the effect of SHE on DC population in skin and ear of DNCB-induced AD mice. When we performed immunohistochemical staining of CD11c+ cells in skin tissues, we observed a large number of CD11c+ cells infiltrated into dermis of skin in DNCB group compared to naïve group (by 2.0 folds, p < 0.005) while it was decreased by DNCB + SHE100 group (by 1.4 folds, p < 0.05) (Fig. 5B). Similarly, in ear tissues an increased number of cells were observed in DNCB group (by 1.6 folds, p < 0.05) compared to that in naïve group, and DNCB + SHE100 treatment decreased the number of CD11c+ cells significantly (by 1.9 folds, p < 0.005) (Fig. 5C) compared to DNCB group. These data suggest a suppressive effect of SHE on the expression of CD11c+ cells in skin and ear tissues of DNCB-induced AD mice.
Sargassum horneri ethanol extracts (SHE) suppressed the infiltration of CD11c+ cells in skin and ear of 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Representative photographs of CD11c+ cells (arrowheads) infiltrated in skin (I–V) and ear (VI–X) are shown (A). The number of CD11c+ cells per site in skin (B) and ear (C) are analyzed. Data are presented as the mean ± standard error. *p < 0.05 and **p < 0.005 indicate significant increase compared to naïve group. †p < 0.05 and ††p < 0.005 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
SHE suppressed infiltration of CD4+ T cells and F4/80 positive cells in skin and ear of DNCB-induced AD mice
Literature indicates that infiltration and distribution of T cells is important for the development of skin inflammation like AD. As expected, a marked infiltration of CD4+ T cells was observed in IHC stained skin sections of NC/Nga mice. However, a large number of T helper cells were infiltrated in DNCB group of AD skin while it could be reduced by the SHE and CJLP133 treatments compared to DNCB group. CD4+ T cells was significantly decreased in DNCB + SHE100 group by 6.0 folds (p < 0.0005) compared to that DNCB group, and a similarly low number of cells infiltrated in DNCB + CJLP133 group compared to DNCB group (p < 0.005). This data suggested that SHE and CJLP133 effectively decreased the CD4+ T cell infiltration in DNCB-induced AD mice (Fig. 6C). Furthermore, a high number of CD4+ T cells were observed in DNCB group while the number of CD4+ T cells seemed to be decreased with SHE treatments in ear tissues of DNCB-induced AD mice (by 1.2 and 1.1 folds, respectively) (Fig. 6E). However, the data revealed that treatment with SHE drastically attenuated infiltration of CD4+ T cells in skin and ear tissues, implying a reduced severity in DNCB-induced AD mice through SHE treatments.

Sargassum horneri ethanol extracts (SHE) decreased CD4+ T cells and F4/80 in skin and ear of 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Representative images of CD4+ and F4/80 positive cells (arrowheads) infiltrated in skin (I–V) and ear (VI–X) are shown (A & B). The number of CD4+ and F4/80 positive cells per site in skin (C & D) and ear (E & F) are analyzed. Data are presented as the mean ± standard error. **p < 0.005 and ***p < 0.0005 indicate significant increase compared to naïve group. †p < 0.05, ††p < 0.005, and †††p < 0.0005 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
IHC staining performed to evaluate the distribution and infiltration of B220+ B cells in AD mice skin tissues also indicated that induction using DNCB increased the infiltration of B220+ B cells in both skin and ear tissues, while treatments with SHE did not affect the infiltration much (data not shown).
Infiltration of immune cells such as macrophages is also known as one of major histopathological changes in AD. When we performed IHC staining for F4/80 to localize and quantify the macrophage population in skin, we identified significantly higher F4/80 cells in DNCB group than in naïve group (9.25 ± 1.3 folds, p < 0.005). However, DNCB + SHE100 and DNCB + CJLP133 groups suppressed the number of F4/80 positive cells compared to DNCB group by 2.4 (p < 0.005) and 3.8 (p < 0.05) folds, respectively (Fig. 6D). Similarly, the number of cells positive for macrophage population in dermis of mice ear was the highest in DNCB group compared to naïve group by 4.8 folds (p < 0.0005). As shown in Fig. 6F, however, treatments of SHE decreased the number significantly. These results indicate that SHE attenuated the macrophage infiltration in skin and ear of AD mice (Fig. 6D & F).
SHE attenuated IL-13 positive cell infiltration in skin and ear of DNCB-induced AD mice
To determine IL-13 positive cells in both skin and ear tissues, we performed immunohistochemical as well. Since IL-13 is known as one of the major cytokines that are expressed in AD, we expected higher level of IL-13 positive cells in DNCB group and we indeed observed 1.75 (p < 0.05) and 2.3 (p < 0.005) folds increases of IL-13 positive cells in skin and ear tissues, respectively (Fig. 7B & C). However, SHE treatments decreased the number of IL-13 positive cells in both skin and ear tissues. DNCB + SHE100 group displayed significant reduction of IL-13 positive cells in skin (by 2.1 folds, p < 0.005) and in ear (by 1.75 folds, p < 0.05) compared to DNCB group. (Fig. 7B & C). These data suggest that SHE suppressed the infiltration of IL-13 positive cells into skin and ear dermis.

Sargassum horneri ethanol extracts (SHE) decreased interleukin 13 (IL-13) positive cells (arrowheads) in skin and ear of 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Representative images of skin (I–V) and ear (VI–X) are shown (A). The number of IL-13 positive cells per site in skin (B) and ear (C) are analyzed. Data are presented as the mean ± standard error. *p < 0.05 and **p < 0.005 indicate significant increase compared to naïve group. †p < 0.05 and ††p < 0.005 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
SHE suppressed collagen deposition in skin and ear of DNCB-induced AD mice
We then performed Masson’s trichrome staining in skin and ear tissues of DNCB-induced AD mice to investigate histological changes occurred in collagen fibers. As shown in Fig. 8, DNCB-induced group showed massive collagen deposition in skin dermis, and the collagen density was significantly increased compared to naïve group (p < 0.0005). However, when the mice were treated with SHE, a considerably lower amount of collagen was seen in skin dermis at DNCB + SHE100 group (p < 0.05) compared to naïve group (Fig. 8B). Similar results were observed in ear tissues as well: collagen deposition was increased in DNCB group by 2.1 folds (p < 0.005) compared to naïve group, but it was significantly decreased at DNCB + SHE100 group compared to DNCB group by 1.5 folds (p < 0.05) (Fig. 8C). This result exhibits that DNCB led to fibrogenesis with massive deposition of collagen fibers while SHE treatment could prevent it.
Masson’s trichrome staining for collagen fiber evaluation in skin and ear of 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Representative photographs of skin (I–V) and ear (VI–X) are shown (A). Black arrows indicate collagen deposited areas in skin and ear dermis. The percentage of collagen density in skin (B) and ear (C) are analyzed. SHE, Sargassum horneri ethanol extracts. Data are presented as the mean ± standard error. **p < 0.01 and ***p < 0.005 indicate significant increase compared to naïve group. †p < 0.05 and ††p < 0.01 indicate significant decrease compared to DNCB group. Scale bars represent: 25 μm.
SHE attenuated DC, T cell, and macrophage population in DNCB-induced AD mice
The percentage of CD45+CD11c+ DCs in spleen of DNCB-induced mice were analyzed by using flow cytometry technique. We observed that the highest percentage of CD45+CD11c+ DC population in DNCB group, significantly higher than that in naïve group (p < 0.005) (Fig. 9B). And treatments with SHE significantly decreased the DC population (by 2.1 and 2.2 folds at 10 and 100 mg kg−1, both p < 0.0005, respectively) compared to DNCB group. Treatment with CJLP133 also decreased the DC population significantly compared to DNCB group (p < 0.005).
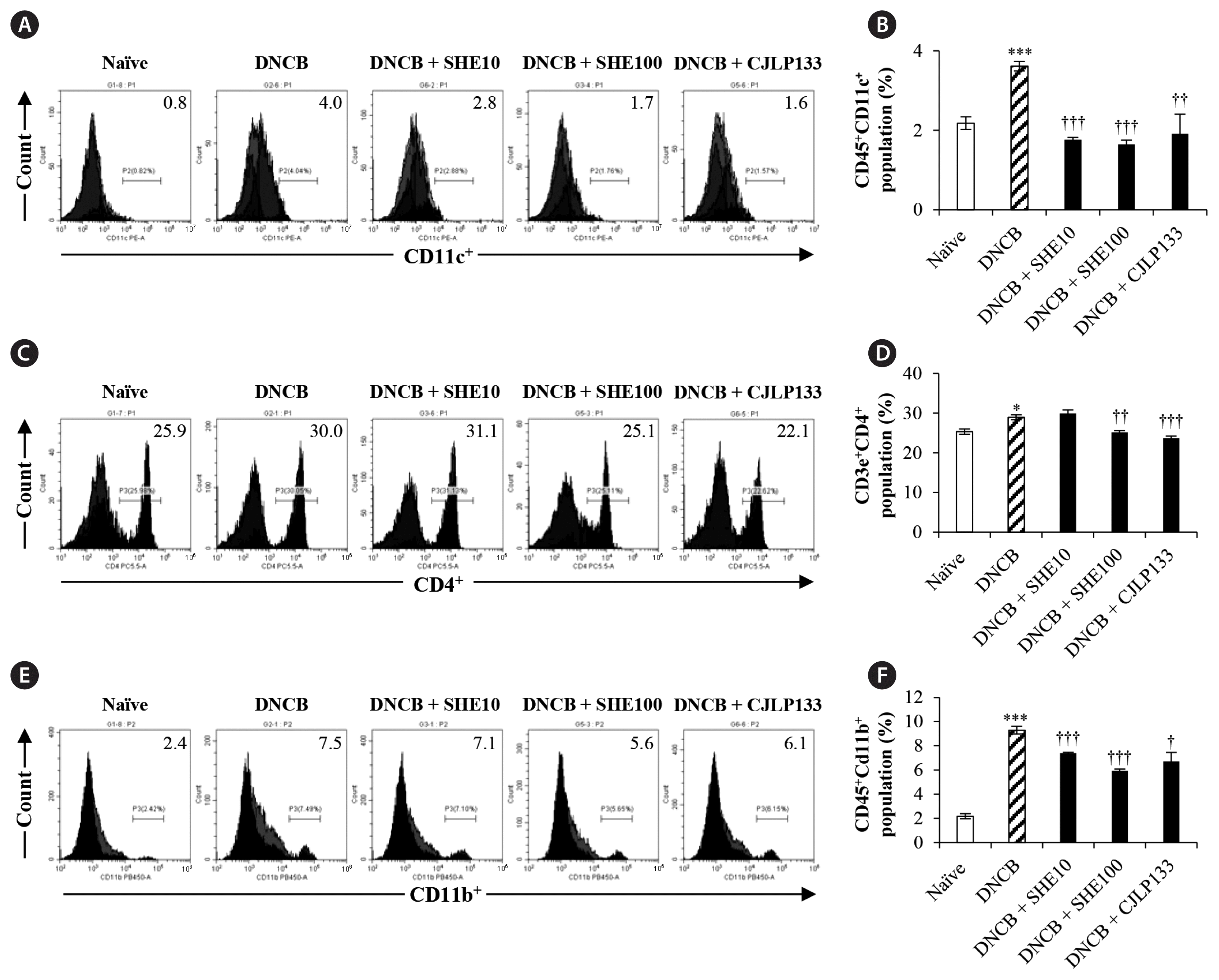
Effect of Sargassum horneri ethanol extracts (SHE) on dendritic cell, T cell, and macrophage populations of spleen in 2,4-dinitrochlorobenzene (DNCB)-induced atopic dermatitis mice. Fluorescence activated cell sorting analysis was performed to evaluate the effect of SHE on populations of CD45+CD11c+ dendritic cell (A), CD4+ T cell (C), and CD45+CD11b+ macrophage (E). Mean values of CD45+CD11c+ dendritic cell (B), CD4+ T cell (D), and CD45+CD11b+ macrophage cell (F) populations are analyzed. Data are presented as the mean ± standard error. *p < 0.05 and ***p < 0.0005 indicate significant increase compared to naïve group. †p < 0.05, ††p < 0.005, and †††p < 0.0005 indicate significant decrease compared to DNCB group.
We also observed that significantly increased CD4+ T cell population in DNCB group relative to naïve group by 1.1 fold (p < 0.05) (Fig. 9D). Treatments with SHE and CJLP133 exhibited significant reduction of cell population: by 1.1 (p < 0.005) and 1.2 (p < 0.0005) folds in DNCB + SHE100 and DNCB + CJLP133, respectively.
In addition, we analyzed CD45+CD11b+ macrophages in spleen of DNCB-induced mice. As shown in Fig. 9E & F, DNCB group exhibited significantly higher cell population compared to naïve group by 4.2 folds (p < 0.0005). More importantly, SHE dose dependently reversed the macrophage population increase in spleen (by 1.2 and 1.5 folds at 10 and 100 mg kg−1, both p < 0.0005, respectively). These results indicate that SHE suppressed the DNCB-induced CD45+CD11c+ DC, CD45+CD11b+ macrophage and CD4+ T cell populations in spleen.
Moreover, when we analyzed B220+ B cell population in spleen of DNCB-induced mice, we observed DNCB induction also increased the B cell population but SHE treatment did not significantly affect the cell population in spleen (data not shown).
DISCUSSION
In our current study, we investigated whether SHE alleviates AD-like skin lesions in NC/Nga mice through suppressing IL-13, a mediator of Th2 immunity. We selected the NC/Nga mouse model since it is known as a spontaneous animal model of AD which resembles human AD closely (Kim et al. 2018). With regard to a recent reference, DNCB is an allergen of low incidence (50%) and late onset in NC/Nga mice (Kim et al. 2018). AD is a major chronic inflammatory skin disease characterized by infiltration of inflammatory cells (Purwar et al. 2006), and Th2-mediated immune reaction has been noted responsible for increased prevalence of AD (Sung and Kim 2018).
Several drugs have been used to cure the inflammatory conditions of AD. Since it was found that synthetic drugs have adverse effects like toxicities, skin atrophy, risk of adrenal suppression, and growth retardation, however, it is imperative to focus on natural functional foods for effective treatments with less side effects (Falguera et al. 2012, Simpson et al. 2017). We recently reported that a decent amount of polyphenols could inhibit chronic inflammation and restore immunity (Herath et al. 2019). Studies also confirmed the effectiveness of polyphenol compounds in the mitigation of AD (Thomas and Kim 2013). More importantly, many studies have recognized that polyphenols from brown algae have anti-inflammation effect in mice (Wijesinghe et al. 2014). Focusing on these factors, we used oral administration of SHE in current study and noted that SHE lowered the number of WBCs in blood increased in AD and also lowered the infiltration of inflammatory cells in skin and ear, implicating that SHE could suppress inflammatory responses. Recent studies implicated that IL-13 contributes to the initial phase of tissue inflammation and also may play a key role in switching to IgE synthesis in AD (Bieber 2010). The activation of cytokines responsible for the NO production could ultimately activate the production of iNOS while increasing cellular NO level and causing erythema and edema during inflammation (Zamora et al. 2000). The iNOS-producing reactions appear mostly in dermal macrophages (Kim et al. 2016). Our results show that SHE reduced the expression of iNOS and COX-2 as noted in the literature that bioactive polyphenolic compounds in brown algae could suppress the COX-2 expression and cell proliferation (Thomas and Kim 2013). Infiltration of eosinophils is a pivotal character of AD and is correlated with symptomatic severity. The literature mentions that an increased number of eosinophils are observed in both sera and lesions of AD patients, and Th2 cytokines mediate the recruitment and function of eosinophils (Kim et al. 2016). Our results signified SHE’s potential in mitigating the infiltration of eosinophils in DNCB-induced AD pathophysiology.
Notably, IL-13 has been considered as a major mediator of CD4+ T cells in acute lesions and peripheral blood of AD (Obara et al. 2002), and it was indeed found to stimulate the activation and recruitment of T cells in skin driving the chronic inflammation in AD (Bieber 2010). The IL-13 activation not only limited drives inflammation in skin but also promotes Th2 cytokine production of lymphocytes in spleen as well (Zheng et al. 2009). In our study, SHE (100 mg kg−1) treatment significantly reduced CD4+ T cells compared to DNCB group in skin and spleen. And it is interesting to note that polyphenols also reduced B cell antibody production and T cell cytokine production during allergen re-exposure (Witzel-Rollins et al. 2019). Furthermore, allergen stimulated DCs cause irritation and scratching in AD, and hence are responsible for Th2-mediated inflammation (Boguniewicz and Leung 2011, Herath et al. 2020). Our results indicate that SHE exerts suppressive effect on DC population in spleen and inhibits CD11c+ cell expression in skin and ear dermis as well. Moreover, DCs prominently express FcɛRI and are responsible for Th2-predominant responses in AD patients (Kim et al. 2016). DCs that migrate to draining lymph node are responsible to present antigenic peptides and to recruit T cells in inflammatory conditions (Oyoshi et al. 1990). It is believed that polyphenol compounds lead the function and maturation of DC (Kim et al. 2007). Macrophage is known to be involved in antigen presentation and phagocytosis, and can stimulate cytokines and chemokines that stimulate collagen synthesis, fibrosis, and capillary growth (Kasraie and Werfel 2013). In AD patients, macrophages appear as dermal mononuclear cells while eosinophils contribute to the inflammatory responses (Kasraie and Werfel 2013). The infiltration of F4/80 in skin tissues and macrophage population in spleen are significantly reduced by SHE treatments as macrophages are able to present pathogen-derived antigens directly to T cells to maintain anti-inflammation (Huang et al. 2000).
IL-13 leads the lesioned skin in AD and its level is highly correlated with disease severity (Bieber 2010). Also, elevated expression of IL-13 could be observed in skin tissues of AD patients. So, it is believed that IL-13 is a key player in the pathogenesis of AD. Moreover, downstream molecular events stimulated by IL-13 are not evaluated yet (Zheng et al. 2009). Interestingly, IL-13 infiltration of skin tissues in our study was decreased by SHE treatments signifying that polyphenols might have suppressed the activation, proliferation, and function of Th2 cells (Chung and Champagne 2009). Moreover, inhibiting IL-13 can reduce the collagen deposition by down regulating matrix metalloproteinase 13 expression and hence overcome the excess collagen deposition resulting in thickened dermis in AD skin lesions (Bieber 2010). Confirming above factors, current study showed that the collagen deposition was diminished in both skin and ear tissues with SHE treatments compared to DNCB group. The fact that IL-13 played the major role of Th2 cytokine and leads skin phenotypes in the mouse model of AD studied in this study closely mirrors the IL-13 dominant phenotypes of human AD by infiltration of CD4+ T cells, eosinophils, F4/80 macrophages involved in skin remodeling including dermal fibrosis in both epidermis and dermis (Zheng et al. 2009). Our results provide an evidence that SHE alleviates the AD-like skin lesions in mice by suppressing IL-13, which is closely linked to inflammatory infiltration of eosinophils, T cells, DCs, and macrophages in AD.
We attempted to identify inflammation-alleviating effects of SHE in AD-like skin lesions in NC/Nga mice through suppressing cytokine IL-13. Since IL-13 has a large impact on inflammatory responses upon exposures to allergens, remarkable changes in their immune responses such as decreased infiltration of inflammatory cells into skin and blood, reduced inflammatory cell population in spleen, and decreased collagen deposition in skin dermis can take place by modulating it. Taken together, findings suggest that oral administration of SHE might be effective in preventing the development of AD-like skin lesions in NC/Nga mice by suppressing Th2-mediated cytokine IL-13.
ACKNOWLEDGEMENTS
This research was supported by the research grant of Jeju National University in 2022. We would like to thank Dr. T. H. Chung for editorial assistance.
Abbreviations
AD
atopic dermatitis
COX-2
cyclooxygenase-2
DCs
dendritic cells
DNCB
2,4-dinitrochlorobenzene
HDM
house dust mites
H&E
hematoxylin and eosin
IHC
immunohistochemistry
IL
interleukin
iNOS
inducible nitric oxide synthase
PBS
phosphate buffer solution
SHE
Sargassum horneri ethanol extracts
Notes
The authors declare that they have no potential conflicts of interest.
SUPPLEMENTARY MATERIALS
Animal model of atopic dermatitis (https://www.e-algae.org).