Incorporating concepts of biodiversity into modern aquaculture: macroalgal species richness enhances bioremediation efficiency in a lumpfish hatchery
Article information
Abstract
Aquaculture is one of the fastest growing food producing sectors; however, intensive farming techniques of finfish have raised environmental concerns, especially through the release of excessive nutrients into surrounding waters. Biodiversity has been widely shown to enhance ecosystem functions and services, but there has been limited testing or application of this key ecological relationship in aquaculture. This study tested the applicability of the biodiversity-function relationship to integrated multi-trophic aquaculture (IMTA), asking whether species richness can enhance the efficiency of macroalgal bioremediation of wastewater from finfish aquaculture. Five macroalgal species (Chondrus crispus, Fucus serratus, Palmaria palmata, Porphyra dioica, and Ulva sp.) were cultivated in mono- and polyculture in water originating from a lumpfish (Cyclopterus lumpus) hatchery. Total seaweed biomass production, specific growth rates (SGR), and the removal of ammonium (NH4+), total oxidised nitrogen (TON), and phosphate (PO43−) from the wastewater were measured. Species richness increased total seaweed biomass production by 11% above the average component monoculture, driven by an increase in up to 5% in SGR of fast-growing macroalgal species in polycultures. Macroalgal species richness further enhanced ammonium uptake by 25%, and TON uptake by nearly 10%. Phosphate uptake was not improved by increased species richness. The increased uptake of NH4+ and TON with increased macroalgal species richness suggests the complementary use of different nitrogen forms (NH4+ vs. TON) in macroalgal polycultures. The results demonstrate enhanced bioremediation efficiency by increased macroalgal species richness and show the potential of integrating biodiversity-function research to improve aquaculture sustainability.
INTRODUCTION
Fish aquaculture can be an efficient way to produce protein for a growing human population, but in many cases it has proven to be unsustainable (Food and Agriculture Organization of the United Nations 2020b). In particular, intensive farming of finfish—usually cultivated in monocultures—has resulted in environmental impacts regarding disease transfer, nutrient pollution, and introduction of invasive species through escapes from open systems installed at sea (Edwards 2015). Enhanced nutrient input causes eutrophication and supports excessive primary production, especially of fast-growing algae species. Eutrophication can cause harmful algal blooms, resulting in species composition changes, diversity loss, and hypoxic conditions and therefore disruption of ecosystem functions (Anderson et al. 2002, Selman et al. 2008, Hale et al. 2016). Accordingly, there are growing calls for the development of better cultivation practices to increase long-term sustainability (Edwards 2015).
Integrated multi-trophic aquaculture (IMTA) is a concept to reduce excessive nutrients released from aquaculture by co-cultivating organisms from different trophic levels (Chopin et al. 1999). Under IMTA, seaweeds as well as other primary producers are incorporated as extracting organisms to take up emitted waste products, such as dissolved nitrogen and phosphorous (Chopin et al. 1999, Chopin 2017). IMTA has also potential for closed recirculation aquaculture systems (RAS) which are increasingly considered as a more sustainable aquaculture practice with less impact on surrounding environments (Bergheim et al. 2009). Recirculation systems are especially used in fish hatcheries and nurseries as environmental parameters and water quality can be better controlled (Murray et al. 2014). However, the accumulation of nitrogen in the form of nitrate is a major factor determining necessary water exchanges in recirculation systems as cost effective mechanisms to reduce the nitrogen concentration are still under development (Goddek et al. 2019). Furthermore, fish aquaculture is not only producing fish for direct human consumption. For example, the exponential growing demand for lumpfish (Cyclopterus lumpus) as a biological treatment against sea-lice in salmon aquaculture has resulted in high demand which heavily relies on hatcheries (Powell et al. 2018).
Nutrient uptake rates of seaweeds have been studied over the last few decades to identify species suitable for efficient uptake of excessive nitrogen and phosphorous from finfish aquaculture (Chopin et al. 1999, Pereira et al. 2008, Abreu et al. 2011, Macchiavello and Bulboa 2014). Generally, macroalgal monocultures have been evaluated based on their nutrient uptake rates and very few studies have implemented a combination of different species into IMTA systems (Ashkenazi et al. 2018). Fast-growing ephemeral species such as Ulva and Porphyra have been recognised as especially good nutrient scrubbers (Carmona et al. 2006, Copertino et al. 2009). Species differ in their preferred nitrogen source, with some seaweeds preferring ammonium, some nitrate, and some showing no preference (Bracken and Stachowicz 2006, Li et al. 2019).
Macroalgal species richness has been shown to enhance total nitrogen uptake as a result of complementary use of different nitrogen sources (Bracken and Stachowicz 2006) and could be applied to improve the bioremediation efficiency of macroalgae in IMTA systems. Since the fundamental work in the early 1990s showed the importance of biodiversity (Naeem et al. 1994, Tilman and Downing 1994), plenty of studies have now demonstrated that biodiversity—as measured by the number of species in a community—tends to enhance resource uptake and biomass production (Cardinale 2011, Gamfeldt et al. 2015, Lefcheck et al. 2015, O’Connor et al. 2017). In particular, resource-use complementarity between different species is commonly associated with enhanced ecosystem functions (Cardinale 2011, Barry et al. 2019). Applying this concept to IMTA or recirculation systems could therefore result in a higher nutrient removal efficiency and biomass production of the co-cultivated species, leading to more efficient resource utilization and reduced production costs.
Niche complementarity and positive diversity effects have been demonstrated in seaweeds (Bracken and Stachowicz 2006, Stachowicz et al. 2008). However, they have rarely been explored in the context of IMTA, where high nutrient concentrations could alter species complementarity and consequently diversity effects, as the complementarity effect (CE) has been observed to be more prevalent under low nutrient regimes (Zhang and Zhang 2006). Therefore, this study investigated whether macroalgal species richness can enhance the bioremediation efficiency in nutrient-rich water from finfish aquaculture. We tested the nutrient removal rate (ammonium, total oxidised nitrogen [TON], and phosphate) from wastewater originating from a lumpfish (Cyclopterus lumpus) hatchery by five macroalgal species in mono- or polycultures. Besides advancing our understanding of the effect of species richness on nutrient fluxes, the study has the potential to improve finfish aquaculture sustainability by enhanced bioremediation efficiency.
MATERIALS AND METHODS
Experimental design
Five macroalgal species (Chondrus crispus, Fucus serratus, Palmaria palmata, Porphyra dioica, and Ulva sp., hereafter referred with genus names only) were chosen for the experiment. Identification of Ulva species based on morphological traits is not reliable and requires molecular methods. However, as biomass for the experiment originated from field collection and as a high number of individuals was needed, reliable species identification based on molecular methods was not feasible for the experimental set up. Therefore, we refer to Ulva using the genus name only. In contrast, while morphological identification to species level is also challenging in Porphyra species, previous molecular analyses support species-level assignment at our local study sites (Knoop 2019). These species were selected based on either their economic value and / or their different life-history strategies. Early succession algae of the genus Ulva are characterised by fast growth and quick nutrient assimilation that are easy to cultivate (Runcie et al. 2003, Ben-Ari et al. 2014). Several companies exist cultivating Ulva on a commercial scale (e.g., ALGAplus, Portugal; Seakura, Israel). Bladed Bangiales (Porphyra and Pyropia) are highly valuable since they are processed into Nori sheets, an essential ingredient for Sushi (Food and Agriculture Organization of the United Nations 2020a). They are also traditionally consumed as laver bread in Wales and combining their high growth and nutrient uptake rates with their economic value raised interest in their cultivation outside of Asia (Kraemer et al. 2004, Pereira et al. 2008). In Europe, interest is high in cultivating local Porphyra species with one of them—Porphyra dioica—being commercially grown at ALGAplus. Chondrus and Palmaria are locally collected for food consumption but have slower growth rates compared to Ulva and Porphyra (Fortes and Lüning 1980, Corey et al. 2014). While Palmaria exploitation is currently mainly based on wild harvest, cultivation protocols are being developed to allow for land-based or open sea cultivation to meet rising demands (Schmedes 2020). Fucus is a relatively slow-growing brown alga with high polysaccharide / alginate content and therefore has potential for polysaccharide extraction (Catarino et al. 2018). Cultivation on commercial scale is being investigated by Algae for Future, Portugal.
Small individuals (5–15 cm) of Chondrus, Fucus, Palmaria, Porphyra, and Ulva were collected from the rocky intertidal at Bracelet Bay, Wales, UK (51°33′58″ N, 3°58′42″ W), in September 2018. Entire individuals were collected including holdfast where possible to minimize stress by tissue damage. Seaweeds were transported to the laboratory in seawater-filled plastic bags and acclimatized to experimental conditions for 48 h. Each treatment monoculture and polyculture plus a control containing no macroalgal biomass was carried out using replicates of n = 6. Polycultures consisted of the combination of four out of the pool of five species. All possible species combinations were formed in order to reduce sampling effects and separate the effects of diversity from any single species (Tilman et al. 1997, Loreau 1998). In total, macroalgae species were combined to five different polyculture groups (Fig. 1, group A = Ulva, Fucus, Chondrus, and Palmaria; B = Porphyra, Fucus, Chondrus, and Palmaria; C = Porphyra, Ulva, Chondrus, and Palmaria; D = Porphyra, Ulva, Fucus, and Palmaria; E = Porphyra, Ulva, Fucus, and Chondrus).
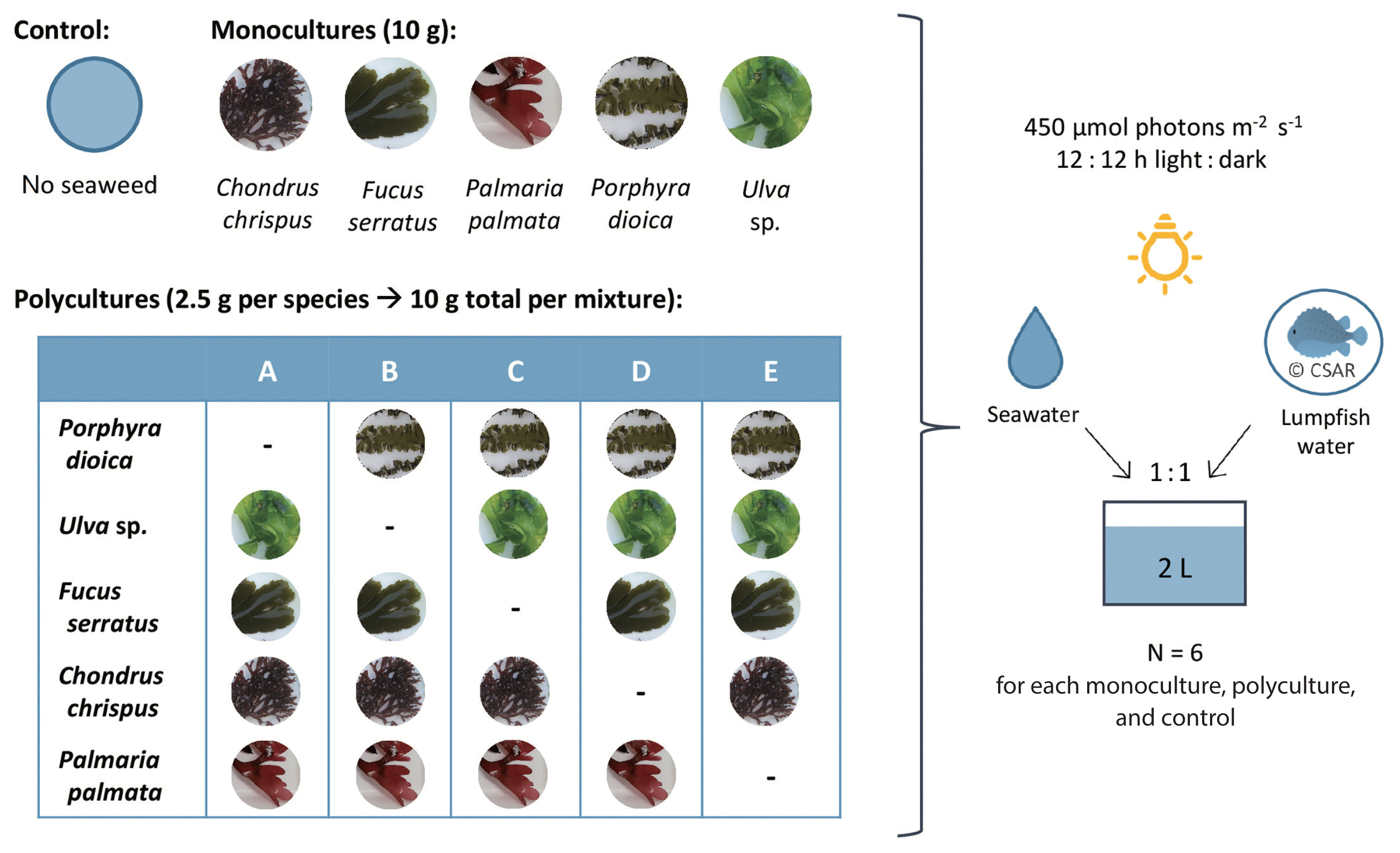
Schematic layout of the experimental design. Controls consisted of only the 1 : 1 mix of seawater and aquaculture water originating from the lumpfish recirculation system without any seaweed biomass. Monocultures contained 10 g fresh weight of seaweed biomass in the aquaculture seawater mix. Polycultures consisted of four out of the five different macroalgal species, with 2.5 g fresh weight of each species resulting in a total of 10 g seaweed biomass in polycultures. Each specific polyculture mix is referred to by the identification letters: A, consisting of Chondrus chrispus, Fucus serratus, Palmaria palmata, and Ulva sp.; B, consisting of Chondrus chrispus, Fucus serratus, Palmaria palmata, and Porphyra dioica; C, consisting of Chondrus chrispus, Palmaria palmata, Porphyra dioica, and Ulva sp.; D, consisting of Fucus serratus, Palmaria palmata, Porphyra dio ica, and Ulva sp.; E, consisting of Chondrus chrispus, Fucus serratus, Porphyra dioica, and Ulva sp.
The experiment was carried out for 9 days in 3-L transparent plastic tanks (28 × 13 × 14 cm), in a total of 66 tanks. Each tank was filled with 2 L diluted lumpfish wastewater (1 : 1) in natural seawater (filtered and ozone treated) due to very high nutrient levels (described in detail under 2.2). The wastewater was sourced from the Centre for Sustainable Aquatic Research (CSAR) lumpfish hatchery (60 m3 recirculation system stocked with 52,000 lumpfish of 7 cm length). CSAR uses natural seawater pumped from Swansea Bay, which is filtered and ozonated before entering the RAS. This water was used for dilution of the experiment too.
Two aeration tubes on one side of each tank created a circular water flow resulting in constant movement of the macroalgae in the tank and even distribution in the water column, ensuring an equal exposure of individual algae to light and nutrients. All selected species can tolerate constant immersion as supported by their presence in local rockpools in the intertidal. Macroalgae were cultivated at a stocking density of 5 g L−1. The concentration is in the range of stocking densities commonly used in macroalgae cultivation (Abreu et al. 2011, Ashkenazi et al. 2018).
Two different experimental designs (additive or substitutive) dominate studies testing the effect of biodiversity. In additive designs, the density of each individual species is constant in monocultures and mixtures, resulting in higher total density with increased species richness (Schmid et al. 2017). In substitutive designs however, total density is kept constant and density of the component species are consequently reduced with increased species richness (Schmid et al. 2017). While additive designs examine the effect of interspecific interactions, the most commonly used substitutive design balances intra- and interspecific interactions and was therefore chosen in the current study (Griffen 2006, Griffin et al. 2009, Gamfeldt et al. 2015). As a substitutive experimental design was chosen, total biomass per tank was the same for mono- and polycultures. In total, following a substitutive design, 10 g of macroalgal biomass was cultivated in monocultures and 2.5 g of each component species (10 g in total) was cultivated in polycultures. Macroalgae were rinsed in natural seawater before experimental set up to remove small crustaceans and epiphytes.
Environmental parameters
Raw wastewater nutrient concentration was very high (1.7 mM TON, 65 μM NH4+, 280 μM PO43−), compared to naturally occurring concentrations which are in the range of 10–40 μM TON, <1–3 μM NH4+, and 1–2 μM PO43− in temperate coastal marine systems (Harrison and Hurd 2001, Voss et al. 2013, Hurd et al. 2014) and close to harmful conditions for fish (Bregnballe 2015). Therefore, the wastewater was diluted 1 : 1, reaching a final concentration of 871 ± 22 μM TON, 32 ± 0.4 μM NH4+, and 141 ± 2 μM PO43. Dilution in the form of a partial water exchange is common practice in recirculation systems to reduce accumulated nutrient concentrations and the here tested nutrient concentrations were in a realistic range within an aquaculture setting. The experiment was set up in a temperature-controlled room at 17°C. Irradiance was supplied at a neutral photoperiod with 12 : 12 h dark : light (L 36W 865 Lumilux; OSRAM, Munich, Germany) at 450 ± 20 μmol photons m−2 s−1. The initial pH and salinity of the water was 8.21 and 30.8, respectively. Temperature, pH, and salinity were measured every morning and afternoon using a WTW Multi 340i Meter with a SenTix Electrode and TetraCon325 Cell (WTW, Weilheim, Germany). Light intensity was measured using a Walz ULM-500 light meter equipped with a US-SQS/L Submersible Spherical Micro Quantum PAR Light Sensor (Walz GmbH, Effeltrich, Germany). Temperature increased throughout the day peaking at 20.5 ± 0.2°C in the evening due to thermal emission of the light sources. As a result, evaporation led to an increase in salinity to 35.3 ± 1.3 after three days. The water was replaced because of the accumulated high salinity, and to remove suspended particles and debris.
Nutrient uptake rates
To calculate the nutrient uptake rates, water samples (15 mL) were taken at 0, 7, 24, 48, and 72 h after the start of the experiment. Water samples were filtered through a 0.2 μm filter and kept at −18°C until analysis of TON, ammonium (NH4+), and phosphate (PO43−) using a Seal Analytical Continuous Flow system (AA3; SEAL Analytical, Norderstedt, Germany). A variant of the methods in Grasshoff et al. (1983) was used. For presentation purposes, nutrient uptake rates are visualized as percentage removal from the water.
Macroalgal biomass
Species-specific biomass was measured after nine days of the start of the experiment, by spinning the seaweed in a salad drier to remove excess water before weighing (de Paula Silva et al. 2008, Ross et al. 2018). Fresh weight was used to calculate specific growth rates (SGR) as follows:
, where FW0 and FW1 are initial and final fresh weights in grams (g), and t0 and t1 are initial and final times in days.
Biodiversity effect
In order to remove the sampling effect and to detect the biodiversity effect on bioremediation, the species-specific average removal of TON, NH4+, and PO43− for each macroalga species was calculated in monoculture. The mean weighted reduction in TON, NH4+, and PO43− was used to calculate the expected nutrient removal according to the species identity in polycultures. The calculated expected value was then compared to the observed value in polycultures to detect the biodiversity effect (Loreau 1998, Griffin et al. 2009). NH4+ removal was compared after 24 h as it was taken up very quickly by macroalgae within the first 24 h. Furthermore, we observed a decrease in NH4+ concentrations in control treatments after 48 h possibly due to NH4+ volatilization because of the strong aeration and therefore water motion (Shpirt 1981, Patoczka and Wilson 1984) (Supplementary Fig. S1). Reduction in TON and PO43− were compared after three days before the water exchange was conducted (Supplementary Figs S2 & S3). The biodiversity effect on biomass production was calculated as described for the nutrient removal. Biomass production was compared at the end of the nine-day period. We also applied additive partitioning to the biomass data to separate CE (resource partitioning or positive interaction effects leading to increased resource use and result in higher biomass) and selection effects (SE; likelihood of including more productive species leading to higher biomass through dominance) from the net biodiversity effect (NE; deviation of polycultures from expected yields based on monoculture observation) (Loreau and Hector 2001). Additive partitioning was calculated after (Loreau and Hector 2001) using Eq. (2):
, where N is the number of species in the polyculture, ΔRY is the difference between expected and observed relative biomass and YM is the observed biomass of a species in monoculture. The horizontal bars above terms indicate the average across the species in polyculture. cov is the covariance between the monoculture biomass of a species and their change in relative biomass in polycultures. Additive partitioning was only applied to biomass data as we did not measure species-specific nutrient uptake rates in the polyculture mixtures.
Statistical analysis
The biodiversity effect—the effect of species richness on TON, NH4+, PO43−, uptake and biomass production—over time was assessed by comparing expected to observed values of the response variables in polycultures using mixed effects models (with replicate as a random effect to account for temporally repeated measures) using the nlme package in R (Pinheiro et al. 2019). TON, NH4+, and PO43− concentrations were log-transformed to meet model requirements. The additive partitioning results of biodiversity effects were analyzed by testing the grand means of the NE, SE, and CE against zero by one-sample t-tests. The effect size of species composition on NE, SE, and CE was tested by one-way ANOVA using the car package in R (Fox and Weisberg 2019) followed by post hoc Tukey tests after requirements for normality and homogeneity were assured. Results are expressed as means ± standard errors unless stated otherwise.
RESULTS
Biomass and specific growth rates
Biomass increased over the experimental period in all species except for Fucus, where a loss in biomass (−0.6% d−1) was observed in all groups, with no difference between mono- or polycultures (Fig. 2). In monocultures, Chondrus, Palmaria, Porphyra, and Ulva grew equally fast between 2 and 3% d−1 (Fig. 2A). Ulva sporulated in at least one replicate per treatment group, except in group A (Chondrus, Fucus, Palmaria, and Ulva) resulting in biomass loss. Therefore, one replicate in the monocultures, groups C, D, and E in Ulva were excluded from the analysis as a result of high biomass loss by tissue disintegration through excessive sporulation.
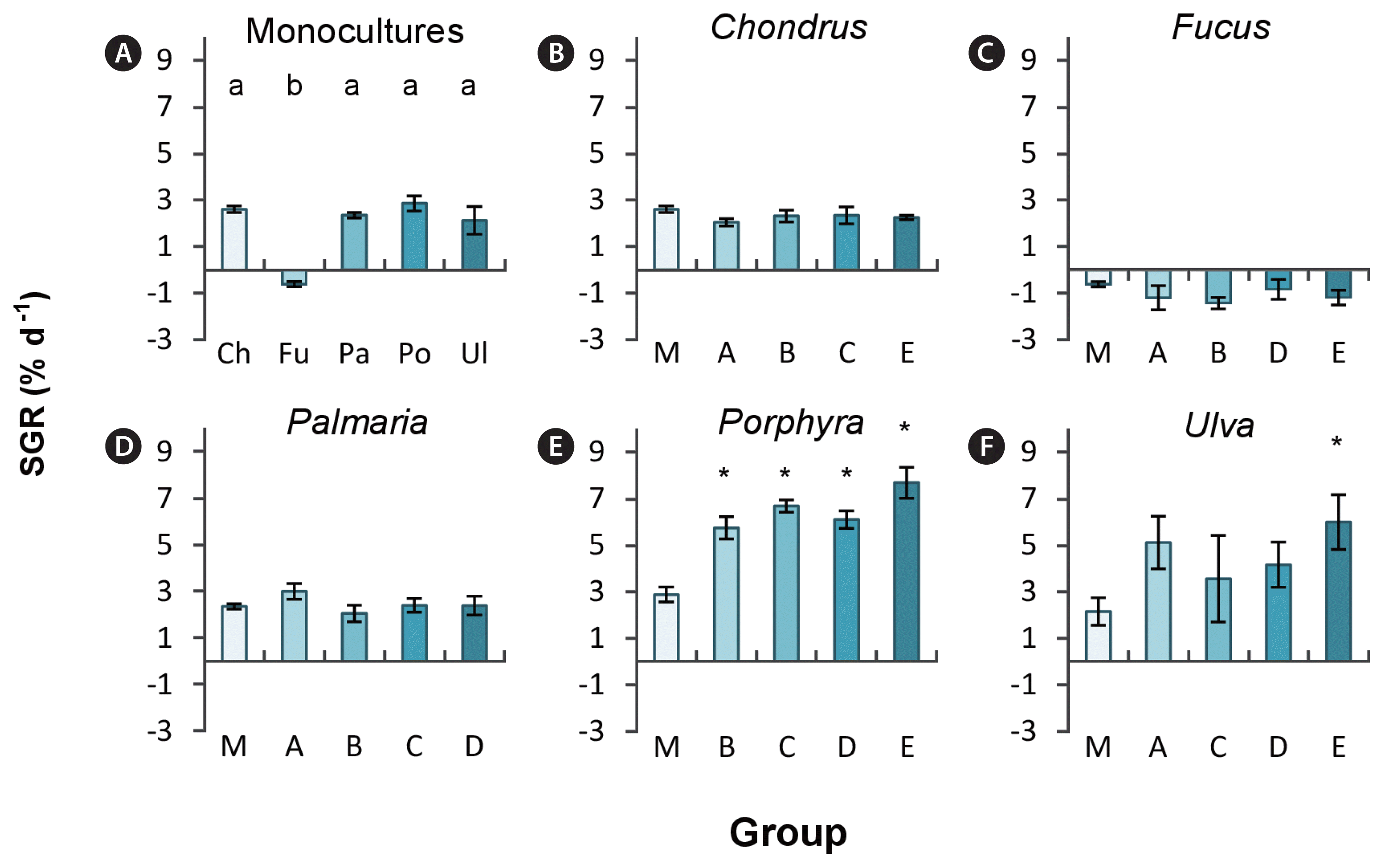
Comparison of specific growth rates (SGR, % d−1) between all macroalgal species (Chondrus crispus [Ch], Fucus serratus [Fu], Palmaria palmata [Pa], Porphyra dioica [Po], and Ulva sp. [Ul]) in monoculture (A) and species-specific comparison of SGR between monoculture (M) and different polycultures (denoted by letters A–E; see Fig. 1 for polyculture compositions) (B–F). Lower case letters in (A) indicate significant differences between monoculture groups (p < 0.05). Asterisks indicate significant differences between polycultures compared to monoculture (*p < 0.05).
In polycultures, the two early succession macroalgae Ulva and Porphyra grew generally better compared to monocultures (Fig. 2E & F), especially in polyculture group E in combination with Chondrus and Fucus. Chondrus and Palmaria grew equally well regardless of mono- or polyculture treatments (Fig. 2B & D).
Species richness enhanced total biomass from on average 5.9 ± 0.2 to 6.6 ± 0.4 g FW L−1 compared to the expected biomass based on observed growth of species in monocultures (χ2(1) = 20.71, p < 0.001) (Fig. 3A), equalling a 11.2% increase of biomass in polycultures. While biomass appeared higher in all polycultures compared to expected, post hoc analysis revealed biomass production was only significantly increased in polyculture group D (lacking Chondrus) and E (lacking Palmaria). Highest biomass (7.2 ± 0.3 g L−1) was observed in group E, consisting of Chondrus, Fucus, Porphyra, and Ulva (Fig. 3B), which was driven by increased SGR of Porphyra and Ulva compared to respective monocultures (Fig. 2E & F).
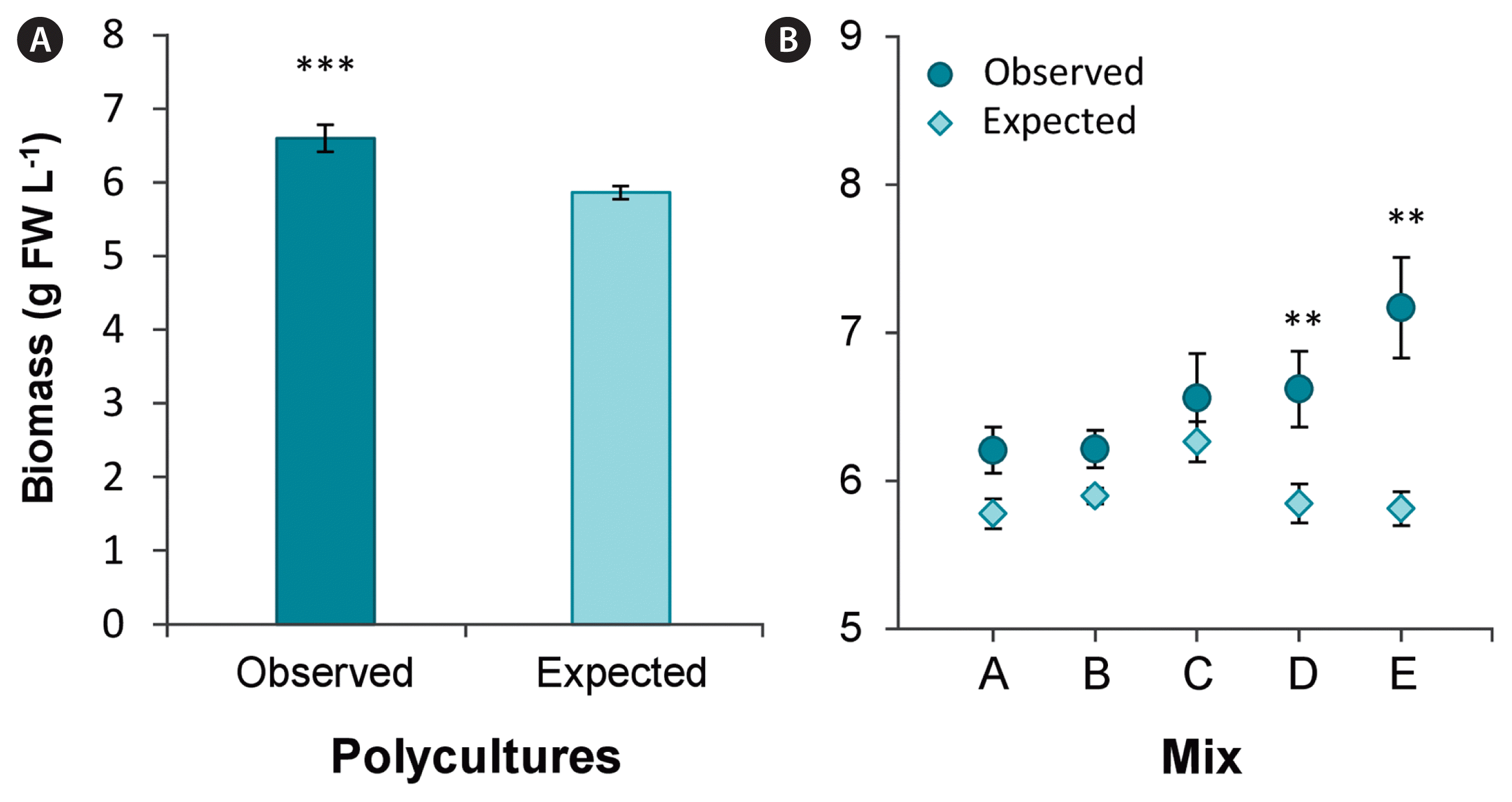
Comparison of observed versus expected biomass after 9 days in polycultures (A) and observed biomass of the different polyculture mixtures (species composition of the polyculture groups are listed in Fig. 1) in comparison to expected biomass (B). Expected biomass values were extrapolated from biomass observations of the respective monocultures. Asterisks indicate significant differences (**p < 0.01, ***p < 0.001).
We next evaluated the extent to which a higher number of species (greater biodiversity) influenced biomass accumulation using the additive partition method. This method separates the overall (net) effect of biodiversity into that attributable to complementarity (where species grow more in mixtures due to interactions such as facilitation and niche partitioning) and selection (where certain species dominate the mixture at the expense of others). Additive partitioning of the biodiversity effect (Fig. 4) showed a significant net effect (t = 4.74, p < 0.01) on biomass, mainly explained by the large CE (t = 4.62, p < 0.01) (Fig. 4B), whereas positive SE only contributed a small fraction to the net effect (t = 3.10, p = 0.04) (Fig. 4C). The species composition of the polyculture only had an effect on the selection effect (F(4,25) = 5.18, p < 0.01).
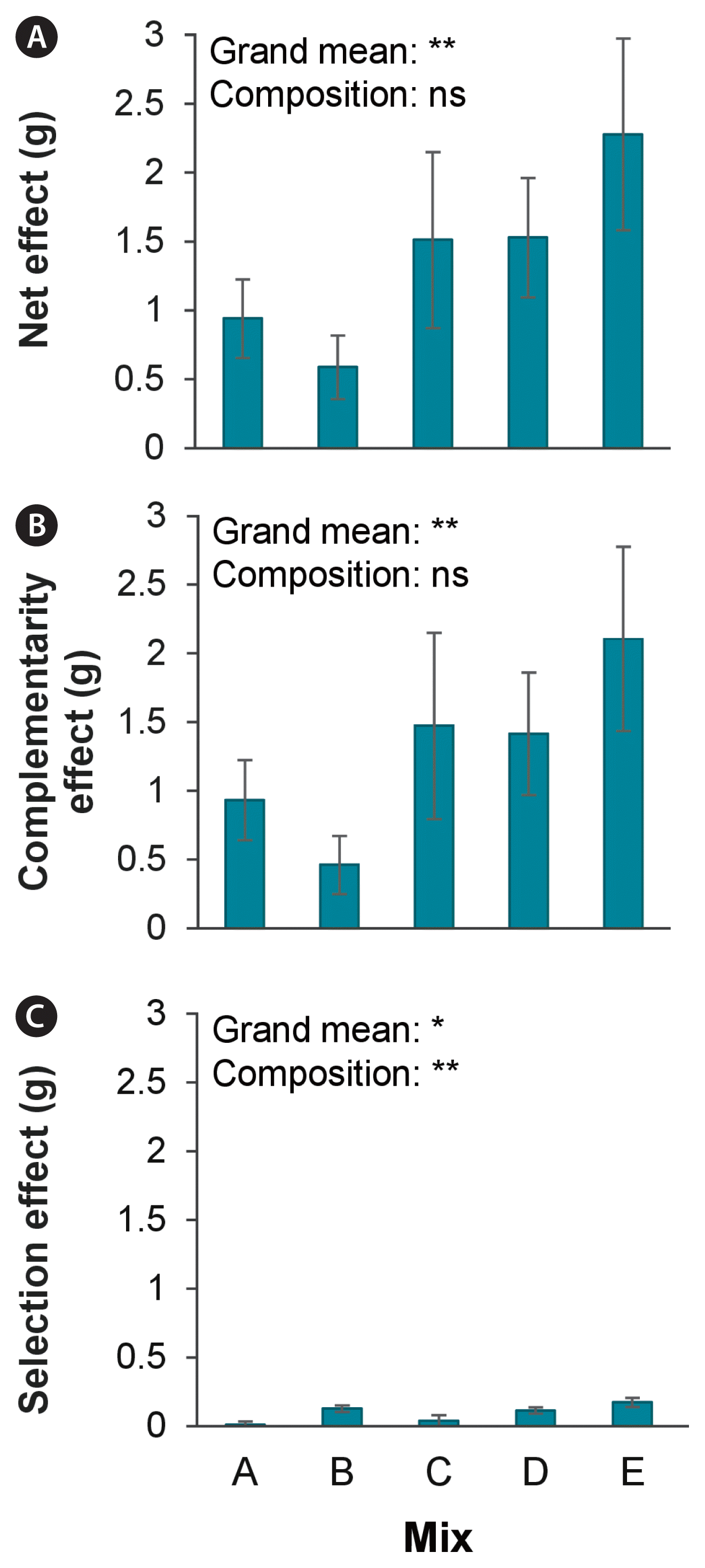
Additive partitioning of biodiversity effects on biomass of macroalgal polycultures dependent on species composition differentiating between net effect (A), complementarity effect (B) and selection effect (C). Species composition of the mixtures are listed in Fig. 1. Asterisks indicate significant effects (*p ≤ 0.05, **p ≤ 0.01; ns, nonsignificant).
Nutrient uptake
Ammonium was effectively taken up within 24 h by most macroalgae whether they were incubated in monocultures or polycultures (Figs 5A & 6A). Only Fucus showed a very low ammonium (5 ± 18%) uptake. Monocultures, excluding Fucus, removed on average 56 ± 4% from initial NH4+ concentrations of 32 ± 0.4 μM, reducing NH4+ concentrations to 14 ± 2 μM within 24 h. Polycultures removed 71 ± 2% of NH4+, reducing NH4+ concentrations to 11 ± 1 μM. While ammonium was effectively taken up within the first 24 h, TON uptake was only noticeable after 48 h (Supplementary Fig. S2).
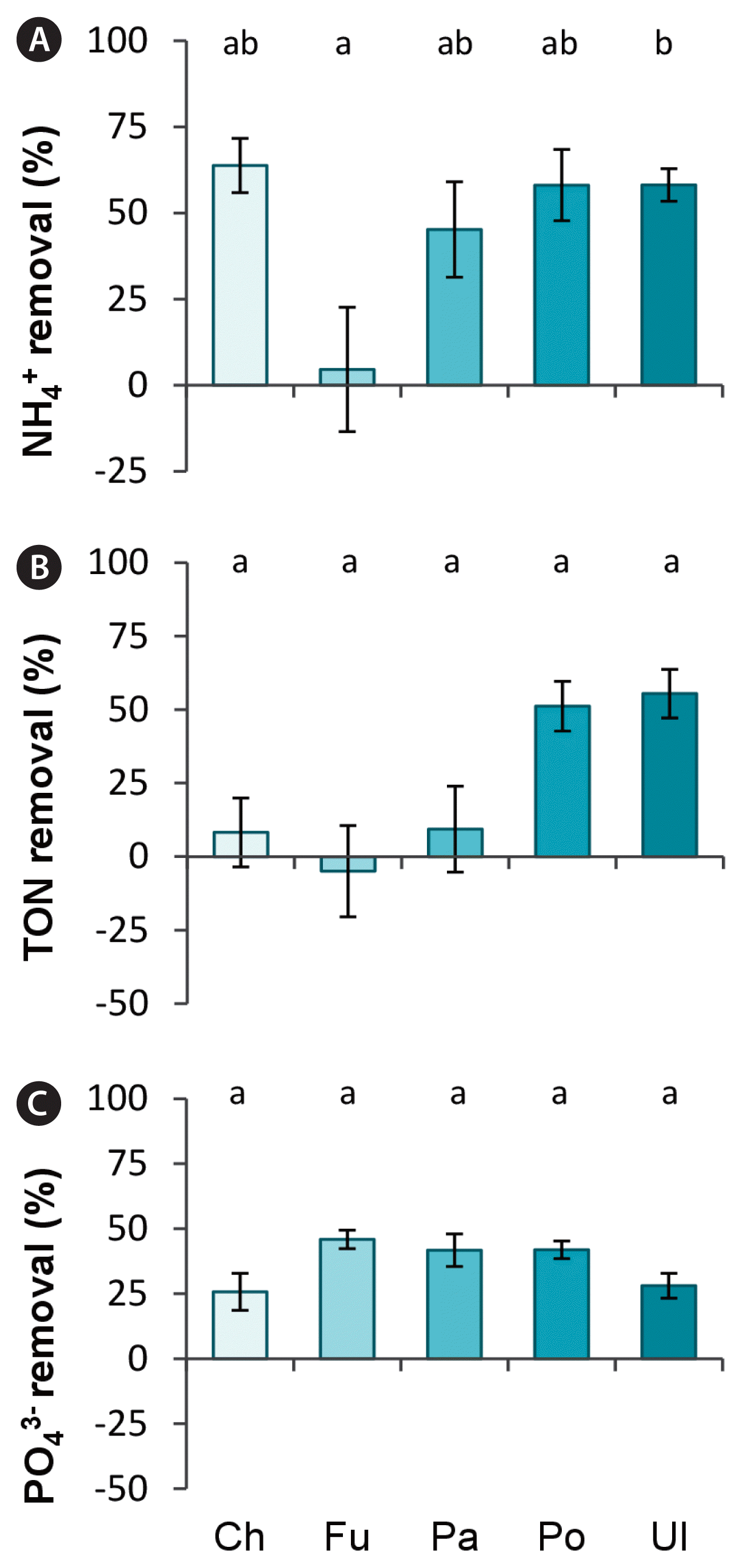
Percentage nutrient removal of ammonium (A), total oxidised nitrogen (TON) (B), and phosphate (C) in different macroalgal monocultures (Ch, Chondrus crispus; Fu, Fucus serratus; Pa, Palmaria palmate; Po, Porphyra dioica; Ul, Ulva sp.) after 24 h for ammonium and 72 h for TON and phosphate. Lower case letters indicate significant differences between groups with p < 0.05.
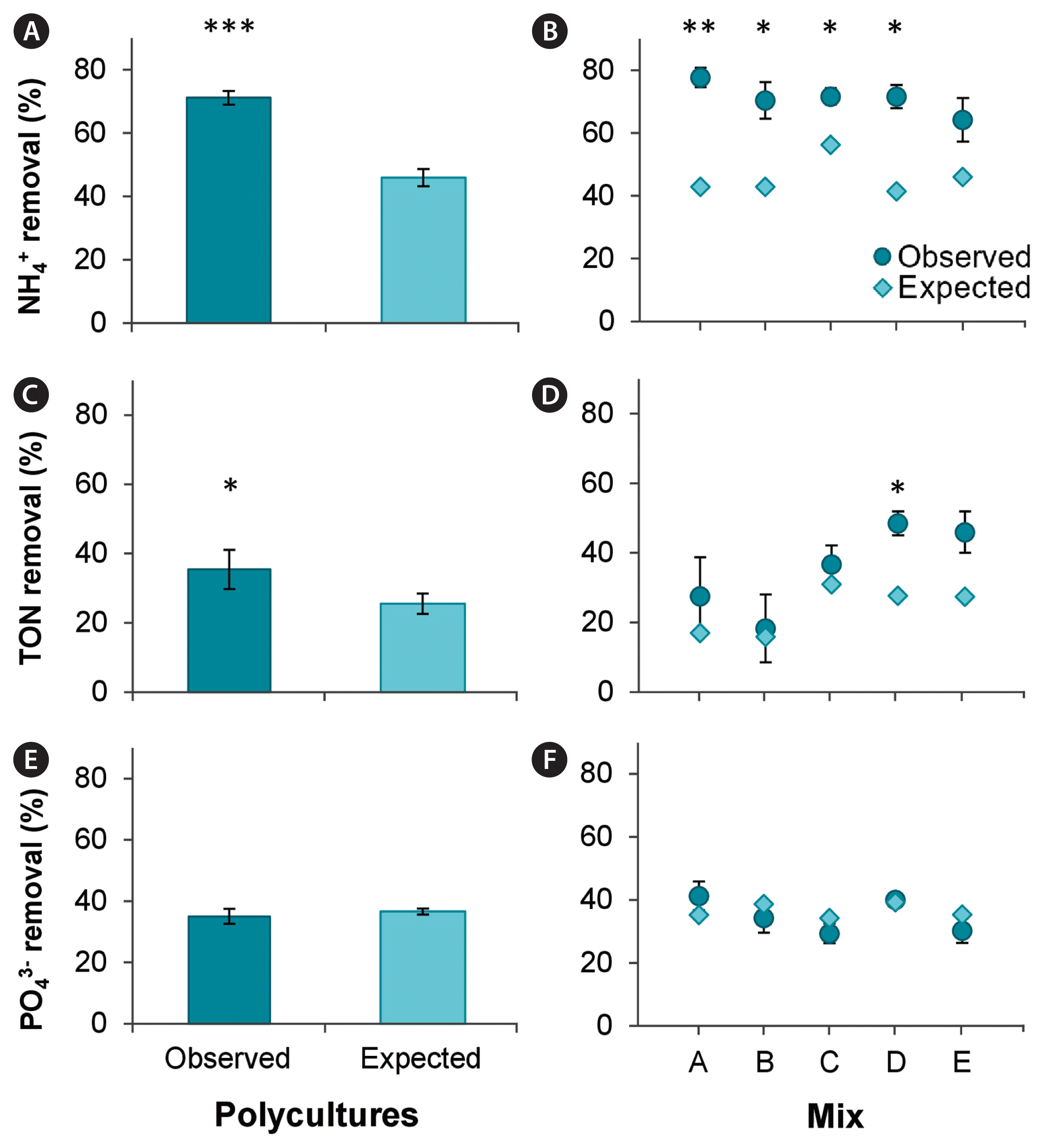
Biodiversity effect on nutrient removal (ammonium [A & B], total oxidised nitrogen [TON; C & D], and phosphate [E & F]). The observed versus expected percentage nutrient removals are compared in grouped polycultures (A, C & E) as well as different polyculture groups (species composition of the mixtures are listed in Fig. 1) in comparison to average expected nutrient removal (B, D & F). Asterisks indicate significant differences compared to expected nutrient removal (*p < 0.05, **p < 0.01, ***p < 0.001).
TON uptake did not significantly differ between species when cultivated in monocultures (Fig. 5B & C). However, Ulva and Porphyra tended to take up more TON (up to 53 ± 2% TON over 72 h) (Fig. 5B), reducing initial TON concentrations from 871 ± 22 μM to 389 ± 55 μM, compared to Chondrus, Fucus, and Palmaria (<5 ± 8% TON over 72 h). Phosphate was taken up equally well between macroalgae whether they were incubated in monoculture (Fig. 5C) or polyculture (Fig. 6C) and was reduced from initial 141 ± 2 μM to 88 ± 3 μM in monocultures and to 92 ± 3 μM in polycultures over the 72 h period.
Species richness enhanced the NH4+ uptake by 25% (Fig. 6A) to 71 ± 2% compared to average expected NH4+ removal of 46 ± 3% (χ2(1) = 40.87, p < 0.001). While the overall effect of species richness on NH4+ uptake was significant (Fig. 6A), post hoc analysis revealed that polycultures A–D significantly increased the NH4+ removal compared to expected NH4+ uptakes (Fig. 6B), whereas no significant effect was observed in polyculture group E (lacking Palmaria). Highest NH4+ removal (78 ± 3%) was observed in polyculture A (lacking Porphyra), reducing the NH4+ concentrations from initial 32 ± 0.4 μM to 10 ± 3 μM. However, NH4+ uptake in polycultures was not significantly higher compared to the most efficient monoculture species Chondrus (64 ± 8%).
Species richness also increased TON removal by nearly 10% (Fig. 6C), resulting in a 35 ± 13% TON reduction from the water in comparison to the expected 26 ± 7% (χ2(1) = 8.27, p < 0.01). However, post hoc results indicated that only polycultures missing Chondrus (group D) resulted in a significantly (p < 0.05) higher TON removal (49 ± 8%) compared to expected (26 ± 7%) values (Fig. 6D). Furthermore, TON removal of polycultures was not enhanced in comparison to the most efficient species in monoculture. Ulva monocultures were the most efficient and removed 55 ± 8% of TON over a three-day period, while polycultures removed on average 35 ± 6% and where therefore 20% less efficient. Of the polycultures, the highest TON removal (49 ± 3%) was observed in polyculture D (lacking Chondrus), which was 7% lower compared to Ulva monocultures.
In contrast, observed percentage PO43− removal did not differ from expected values in any of the polycultures (Fig. 6E & F) and species richness did not improve PO43− remediation. Polycultures removed PO43− equally well with on average 37 ± 2%.
DISCUSSION
In this study, we assessed whether concepts of biodiversity—ecosystem functioning research can be integrated into aquaculture to reduce the environmental impact by enhancing bioremediation using increased macroalgal species richness. The presented results show enhanced biomass production and bioremediation (NH4+ and TON) with increased macroalgal species richness under an IMTA concept. Therefore, incorporating macroalgal species richness into finfish aquaculture might have potential to enhance finfish aquaculture sustainability.
Biomass
Similar growth rates were observed for all monocultures except Fucus, where a loss in biomass was observed. Growth rates of the fast-growing opportunistic species Porphyra and Ulva, in monocultures, were not higher compared to Palmaria and Chondrus resulting in similar productivity across species. This observation changed under the polyculture treatments, with a positive effect of macroalgal species richness on biomass production. The positive relationship between biodiversity (species richness) and productivity has been observed in numerous previous studies (Cardinale et al. 2006, O’Connor et al. 2017). Overyielding of high biodiversity (high species richness) communities or treatments can be explained by resource partitioning, abiotic facilitation, and biotic feedbacks (Barry et al. 2019). The strong detected CE suggests that the observed overyielding was a result of resource partitioning or positive species interactions (Loreau and Hector 2001). Overyielding in polycultures was mainly driven by higher growth rates of the two fast-growing early succession algae Porphyra and Ulva compared to respective monocultures. In particular, Porphyra grew consistently better in polyculture compared to monocultures. These results are in alignment with a previous study which demonstrated that species identity was a major driver of biomass production under increased macroalgal species richness (Bruno et al. 2005). Similar to our results, this study (Bruno et al. 2005) observed higher productivity in the opportunistic Ulva lactuca in mixtures compared to monocultures. The enhanced growth of Porphyra and Ulva with increased species richness can be a result of reduced intraspecific competition following the substitutive design. Because Porphyra, and to a certain extent Ulva grew faster in polyculture, compared to their respective monocultures, we conclude that the higher observed biomass is a consequence of the CE (Tilman et al. 1997).
Besides the positive effects on growth on the two opportunistic species Ulva and Porphyra, species richness did not influence growth in Chondrus and Palmaria. Chondrus and Palmaria are slower growing in comparison to Ulva and Porphyra (Sharp 1987, Corey et al. 2014). The period of nine days, during which the experiment was conducted, might have been too short to detect any differences in growth between monocultures and polycultures in the slower-growing species. Fucus was the only species that did not grow in the experiment, regardless of whether cultivated in monoculture or polyculture. Eutrophication has been linked to declining Fucus populations, most likely as a result of combined direct and indirect effects from nutrient pollution, decreased water quality, enhanced grazing and epiphyte cover, resulting in reduced fitness (Nilsson et al. 2004, Vahteri and Vuorinen 2016). Temperature and light intensity within our experimental setting were comparable to natural local conditions and therefore the high nutrient concentration could have caused the tissue disintegration in Fucus. Additionally, the here observed growth rates for Palmaria and Chondrus were rather low compared to other studies where growth rates of up to 8% have been observed for Chondrus (Roleda et al. 2004). This suggests that not all macroalgal species tolerate high nutrient concentrations associated with IMTA and highlights the need for further research establishing nutrient tolerance thresholds for species of interest and identifying species with high nutrient tolerance. Furthermore, nutrient concentrations will vary greatly within a fish cultivation system depending on water exchange rates, fish density, and feeding regime. Therefore, the here observed results need to be critically assessed under different nutrient concentrations or ideally integrated into a running system for an extended period of time to estimate productivity and bioremediation under dynamic nutrient conditions.
Bioremediation
Macroalgal monocultures, except Fucus, effectively removed NH4+ with Chondrus and Palmaria showing a clear preference for NH4+. Furthermore, Porphyra and Ulva monocultures effectively removed TON as well. However, while the uptake of NH4+ was observed very quickly after the start of the experiment within the first 24 h, TON removal was only apparent after a longer incubation period of three days, indicating a preference for NH4+. The delay in TON uptake in comparison to the immediate NH4+ removal can be explained by different uptake mechanisms as NH4+ is taken up by passive diffusion, while NO3− (the major component of TON) is taken up actively, requiring energy (Roleda and Hurd 2019). Furthermore, high NH4+ concentrations can inhibit the NO3− uptake and can even have toxic effects in higher plants and microalgae—although the impact on macroalgae is not well studied (Britto and Kronzucker 2002, Collos and Harrison 2014, Roleda and Hurd 2019). In macroalgae, NH4+ concentrations of 10 μM have been observed to inhibit TON uptake (Hanisak and Harlin 1978). Initial NH4+ concentration in our set up was very high (32 μM) and could therefore be a reason why TON was only taken up once NH4+ concentration was reduced.
Macroalgal species richness clearly enhanced the bioremediation efficiency of NH4+. Furthermore, macroalgal species richness had a positive effect on TON removal. However, the effect was less clear compared to NH4+ as, while we observed an overall positive effect of species richness on TON removal, post hoc analysis revealed that TON removal was only more efficient than the monocultures in group D. Resource partitioning and specifically the complementary use of different nitrogen sources could be the underlying mechanism explaining the higher nitrogen removal under polyculture conditions (Barry et al. 2019). Complementary use of different nitrogen sources was also the suggested reason for higher nitrogen uptake with increased species richness observed by Bracken and Stachowicz (2006). Resource partitioning of chemical forms of nitrogen has also been observed in grasslands when polycultures contained a dominant species that quickly took up the best available nitrogen source (Ashton et al. 2010). This could be a potential explanation for the observed biodiversity effect on nitrogen uptake in polyculture treatments. Especially Porphyra and, to an extent, Ulva, grew better in polycultures and both took up NH4+ as well as TON, while Chondrus and Palmaria showed a clear preference for NH4+. In contrast to biomass, we cannot state which species accounted for the higher than expected nitrogen removal in polycultures as we did not measure individual uptake rates of the different species in polyculture. We can only speculate that, since Porphyra and Ulva grew faster in polyculture treatments, their enhanced performances in mixture were most likely driving the enhanced nitrogen removal in high species diversity treatments.
Integrating macroalgal biodiversity into IMTA
Our study showed that macroalgal biodiversity can increase the bioremediation efficiency if integrated into fish aquaculture. Even if polycultures were not more efficient with regard to bioremediation than the most efficient monoculture, in our case Ulva, the incorporation of different macroalgal species into finfish aquaculture has further benefits. Besides improving water quality in terms of nutrient load, pH, and oxygen concentration, macroalgae are associated with species-specific microbial communities (Lachnit et al. 2009). Depending on species combination, this could alter bacterial compositions towards beneficial microbial communities. However, more research is needed to investigate the effect of co-cultivated macroalgae onto the microbial community in aquaculture environments. Furthermore, macroalgal extracts can have strong antibacterial or antiviral activity against pathogens commonly observed in aquaculture (Stabili et al. 2019). Incorporating the macroalgal biomass into the feed of co-cultivated species could increase the sustainability of the cultivation site through direct nutrient cycling and simultaneously enhance health and fitness of co-cultivated organisms.
Diseases are also an emerging problem in commercial macroalgae cultivation as a result of the fast growth of the industry, mainly following a monoculture approach with limited genetic diversity (Gachon et al. 2010, Hafting et al. 2015). Increasing cultivated macroalgal species richness and genetic diversity therefore could reduce the susceptibility against diseases and should be investigated as an effective biological tool to reduce disease outbreaks allowing to maintain stable macroalgal cultures in the long-term (Boyer et al. 2009, Gachon et al. 2010). However, the possibility of transmitting diseases between species should be considered under a polyculture approach and requires further investigation. Another point to consider is the intended application of seaweed biomass originating from an IMTA system. For example, food safety standards need to be addressed in case the biomass is meant for human consumption.
The here observed enhanced growth of some macroalgal species with increased species richness resulted in higher yields and therefore in higher efficiency in biomass production. Currently, mainly fast-growing but usually low-value macroalgal species, such as Ulva, are recommended as good candidates for bioremediation (Tremblay-Gratton et al. 2018). Combining slow growing but highly valuable species with fast-growing species could allow for product diversification, accompanied by higher bioremediation and biomass production. However, combined species could also have negative effects on each other (Friedlander et al. 1996) and therefore, allelopathic effects need to be evaluated between combined species.
This study demonstrates promising results that can improve the sustainability of aquaculture systems. However, it was a first investigation and the setup should be tested at a larger scale incorporated into a flow through system as a next step to verify these findings under a constant re-supply of nutrients. Further experiments should investigate best possible species to be integrated in such a system, which species combinations are most favourable and how large such a bioremediation tank would have to be to reduce a significant amount of excessively produced nutrients while adding a possible profit to the aquaculture system. Furthermore, it may not be feasible, on a larger scale, to have a mixture of different macroalgal species in one tank as it would require sorting of the biomass upon harvesting, unless a mixture of different species is favorable for example as a feed additive. Therefore, a tank design with different compartments should be investigated, allowing easy harvesting of the biomass while enabling a homogeneous mixture of the nutrient rich water. This could be combined with optimizing growth conditions in terms of light requirements for the combined species, potentially resulting in even higher growth rates and nutrient uptake rates. Another aspect to consider is the supply of initial seaweed biomass. Harvesting large quantities from wild populations should be avoided and selected species should be considered based on available cultivation protocols.
Previous extensive research has shown that biodiversity tends to enhance ecosystem functioning in various ecosystems. However, this key ecological concept has rarely been integrated into aquaculture practices as verification was required under eutrophic conditions to investigate whether high nutrient levels would inhibit enhanced nutrient uptake and productivity. Here, we have demonstrated that macroalgal species richness can enhance biomass production as well as bioremediation efficiency of NH4+ and TON when cultivated in wastewater from finfish cultivation, advancing the concept of IMTA. Overall, the results demonstrate the possible improvement of finfish aquaculture sustainability by enhanced bioremediation efficiency with increased macroalgal species richness.
ACKNOWLEDGEMENTS
The study was funded by a Knowledge Economy Skills Scholarship (KESS)–a pan-Wales higher level skills initiative led by Bangor University on behalf of the HE sector in Wales–partially funded by the Welsh Government’s European Social Fund (ESF) convergence programme for West Wales and the Valleys and the Pembrokeshire Beachfood Company. We would further like to thank the team of the Centre for Sustainable Aquatic Research at Swansea University for their technical support.
Notes
The authors declare that they have no potential conflicts of interest.
SUPPLEMENTARY MATERIALS
Percentage ammonium concentrations in the control, monocultures, and species mixture treatments over three days relative to initial concentrations (https://www.e-algae.org).
Percentage total oxidised nitrogen (TON) concentrations in the control, monocultures, and species mixture treatments over three days relative to initial concentrations (https://www.e-algae.org).
Percentage phosphate concentrations in the control, monocultures, and species mixture treatments over three days relative to initial concentrations (https://www.e-algae.org).