INTRODUCTION
Multicellularity is one of the most prevalent evolutionary innovations and has evolved multiple times (Miller 2010). It has been believed that the physical adhesion of cells to build a larger unit enables the organism to survive better in ever-changing environments and affords a better defence against various stressors (Boraas et al. 1998, Justice et al. 2008, Lyons and Kolter 2015, Kuzdzal-Fick et al. 2019). Modern models for the evolution of multicellularity are based on the concept of division of labor, typically between vegetative and reproductive cells (Ispolatov et al. 2012). The intercellular transfer of nutrients and signals is necessary to achieve division of labor between cells. Multicellular eukaryotic organisms have evolved gap junctions or plasmodesmata as intercellular routes for cytoplasmic substances and signaling molecules between adjacent cells (Nagasato et al. 2017). Plasmodesmata ensure a continuum of cytoplasmic communication while regulating the transport of macromolecules between cells (Lee 2014).
Plasmodesmata have been well documented in land plants, some green algae, and analogous structures in brown algae (reviewed in Robards and Lucas 1990, Raven 1997). Some plasmodesmata contain desmotubules, which are tubular strands of connecting endoplasmic reticulum (Ding et al. 1992). Small molecules, such as inorganic ions, hormones, metabolites, as well as macromolecules, including RNAs and proteins, are transported through plasmodesmata (Lucas et al. 1995, McLean et al. 1997, Kim et al. 2005). Early studies using microinjection of fluorescent tracers showed that the transferable molecular size limit across plant plasmodesmata is approximately 1–3 kDa (Erwee and Goodwin 1983, Goodwin 1983, Terry and Robards 1987, Kempers et al. 1993), but accumulating evidence showed that some plants adjust the sizes of plasmodesmata apertures in response to biotic and abiotic stress, thereby regulating the size of molecules that are transported between cells (Lee 2014). The transport of green fluorescent proteins (GFP) with a molecular size of 27 kDa has been observed to move to neighboring cells through plasmodesmata (Liarzi and Epel 2005). The permeability of plasmodesmata also changes when the intracellular calcium level is different between adjacent cells (Su et al. 2010).
Red algae are a group of mostly multicellular organisms that evolved multicellularity independently (Gawryluk et al. 2019). Red algae are different from other multicellular plants and algae in lacking any structures similar to plasmodesmata (Bouck 1962). Instead, red algae have a pit-connection between cells. Pit-connections are formed between two daughter cells by an incomplete centripetal infurrowing of the plasmalemma. Cytokinesis is therefore incomplete, leaving a pore or connection, which is subsequently ‘plugged’ with various proteinaceous, or other, materials (the pit plug) (e.g., Pueschel 1990). Pit-connections do not permit cytoplasmic continuity between cells as the pit plug tightly occludes the connection. Variation in pit plug morphology is seen in the number of cap layers on either end of the plug core, the morphology of the outer cap layer, if present, and the presence or absence of a cap membrane across each end of the pit plug (see Pueschel 1990). Pit plugs without surrounding membranes were observed in particular cells of female gametophytes connected to the nutrient-dependent carposporophyte (Broadwater and Scott 1982, Tsekos and Schnepf 1985). It has long been proposed that this type of pit-connection may serve as a “transfer connection” interconnecting vegetative and reproductive cells and enhancing nutrient transport as it becomes more pronounced in reproductive cells after fertilization (Wetherbee 1979). However, no experimental evidence showed that pit-connection, the only physical connection between cells, acts as conduits between these specialized cell types.
Indirect evidence of intercellular transport between red algal cells has been reported in Griffithsia sp. (Koslowsky and Waaland 1984, 1987). A cytoplasmic incompatibility factor was found to move by diffusion from a hybrid somatic cell to adjacent cells, but the authors did not claim pit-connections as the intercellular passage (Koslowsky and Waaland 1987). Electric coupling was observed between Griffithsia cells, but a dye coupling or movement of cobalt ions through pit plug was not observed (Bauman and Jones 1986). Thus, no experimental evidence unequivocally shows that pit-connections act as conduits between vegetative cells.
In this study, we hypothesized that the pit-connection is a junction that the intercellular transport occurs between vegetative cells of red algae and is functionally analogous to plasmodesmata. We examined the movement of macromolecules of various sizes across the pit-connection by using microinjection of fluorescent tracers in Griffithsia monilis cells.
MATERIALS AND METHODS
Plant material and laboratory culture
A male strain of G. monilis was provided by Prof. J. A. West in 2008 and kept in laboratory culture until use. Cultures were maintained in IMR medium (Klochkova et al. 2006) at 15°C, 14 : 10 h LD cycle and 30 μmol photons m−2 s−1 flux density.
Preparation of recombinant green fluorescent protein
Recombinant plasmids for GFP expression were constructed by ligating the polymerase chain reaction product into pCold II DNA vector (TaKaRa, Shiga, Japan) as described by Nagasato et al. (2017). Plasmids were digested with XbaI and SacI enzymes. BL21 competent cells (TaKaRa) were transformed with the recombinant plasmids. Protein expression and purification (His TALON Gravity Column purification kit; Clontech Laboratories, Mountain View, CA, USA) were carried out according to the manufacturer’s protocols.
Microinjection of fluorescent probes
Filaments of G. monilis were embedded in 1% low-melting-point agarose in seawater (Type VII; Sigma Chemical Co., St. Louis, MO, USA) and mounted on slides before microinjection. Micromanipulators consisting of a coarse manipulator (MN-4; Narishige, Tokyo, Japan) and a three-axis joystick oil hydraulic micromanipulator (MO-202; Narishige) were coupled to an Olympus IX71 inverted microscope (Olympus, Tokyo, Japan). Fine glass needles were produced by pulling glass capillaries (GDC-1; Narishige) with a microcapillary Faller MCF-100 (Nepa Gene Co., Ltd., Tokyo, Japan). Fluorescein isothiocyanate-dextran (FD; Invitrogen-Molecular Probes, Carlsbad, CA, USA) of four different molecular sizes (3, 10, 20, and 40 kDa) and recombinant green fluorescence protein (rGFP) were used for injection experiments. An injection solution composed of 0.2 mM KCl, 10 mM HEPES, and 0.55 M mannitol at pH 7.0 (Farnham et al. 2013) was mixed with the fluorescent tracers to give a final tracer concentration of 1 mg mL−1. Along with the tracer, ionic liquid (SW-25; Nepa Gene Co., Ltd.), a laser absorbent, and a UV absorbent (WF-25; Nepa Gene Co., Ltd.) were added to the glass needle. Micromanipulation was performed by a laser thermal microinjector LTM-1000 (Nepa Gene Co., Ltd.). We injected 10–20 cells in different plants in each experiment (3, 10, 20, and 40 kDa). The intercellular movement of the fluorescent tracers was observed under an epifluorescence microscope (BX50W-FL; Olympus) equipped with differential interference contrast. Images were captured using a CCD camera (AxioCam; Zeiss, Jena, Germany) over a time course, and digital images were converted with AxioVision 4.8 (Zeiss) and Photoshop Elements 10 (Adobe Systems, San Jose, CA, USA) for the final image presentation.
RESULTS
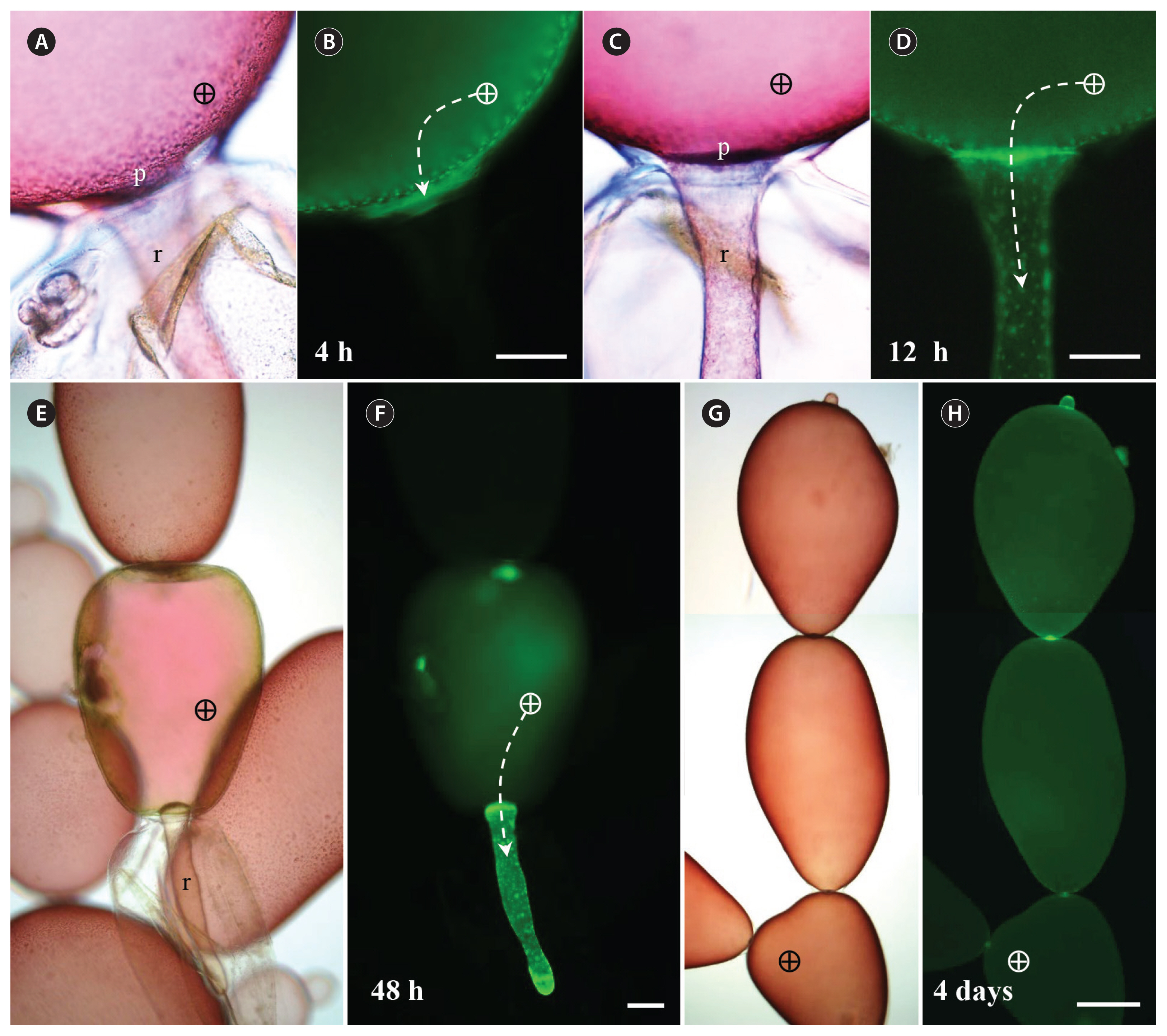
We found that macromolecules, up to 20 kDa, can be transported between different cells in G. monilis (Fig. 1). In most cases, tracers were only injected into the parietal cytoplasmic layer of the cell as cell collapse occurred when the microinjector needle was pushed deep into the central vacuole. FD began to diffuse from the injected site to fill the entire cell within 30 min (Fig. 1A & B). The apical parts of the cell and the pit-connection area accumulated more tracer than other parts of the cell (Fig. 1B). At this time, strongly fluorescing spots were observed within the parietal cytoplasm which appears to be a result of refraction occurring when the fluorescence of the tracer passes through the dense nucleus (Fig. 1B). Transfer of FD to adjacent reproductive cells was observed 1 h after injection (Fig. 1C & D). Spermatangial reproductive cells, which develop on the shoulder of vegetative cells, became fluorescent faster than adjacent vegetative cells (Fig. 1D). Transfer of FD to adjacent apical cells was observed in 2 h (Fig. 1E & F). FD accumulation was higher in adjacent spermatangial cells than in the adjacent vegetative cells also 3 h after injection (Fig. 1G & H). FD was in turn transmitted apically over cells 6 h after injection (Fig. 1I–K).
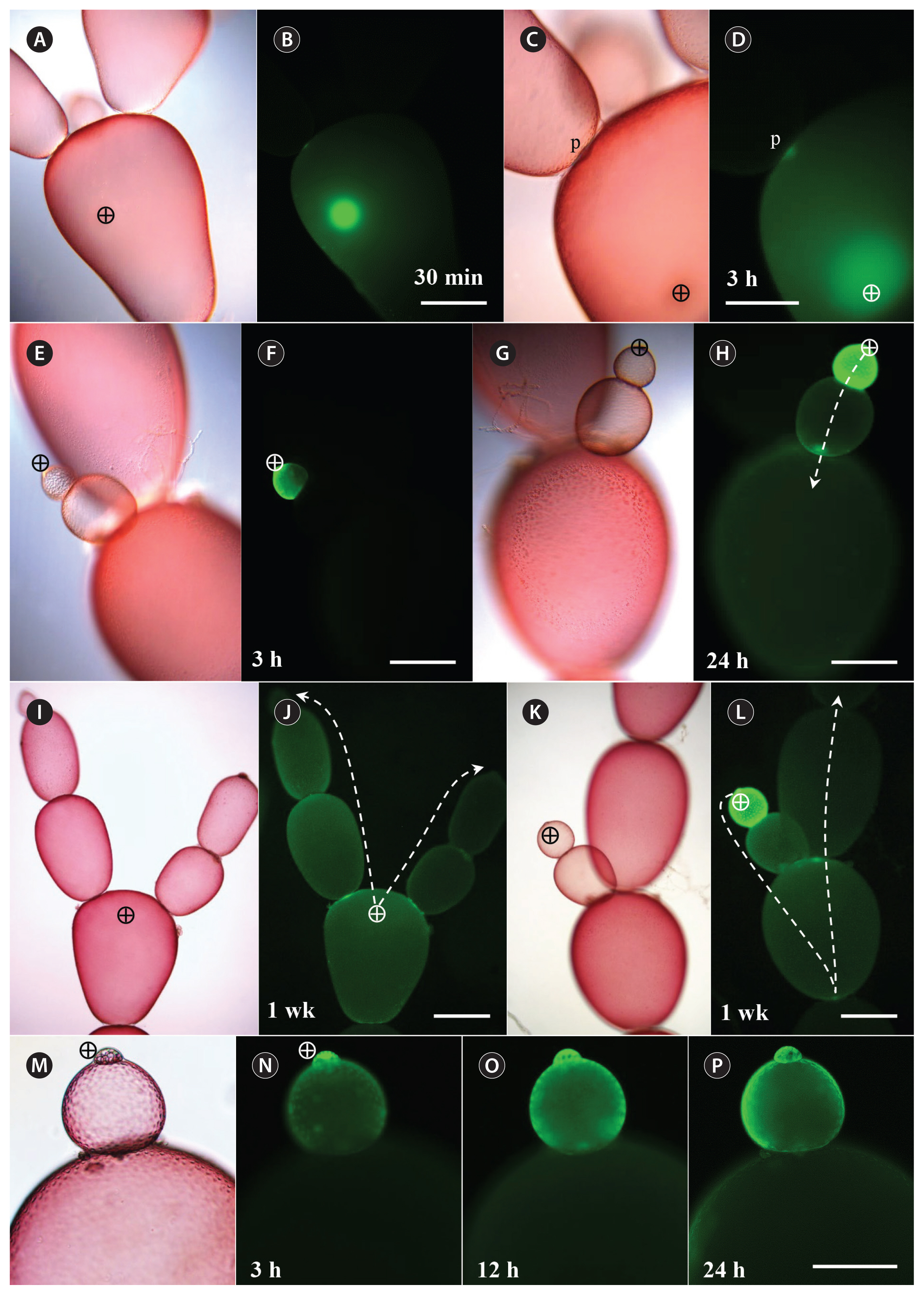
To observe whether the FD spontaneously leaked into the wound site, the filaments were cut and 3 kDa FD was injected into the basal cell adjacent to the wound (Fig. 2). FD transport to regenerated rhizoidal cells occurred, but at a much slower pace; no FD was detected after 4 h (Fig. 2A & B), but it was present throughout the rhizoid by 12 h (Fig. 2C & D). When the FD injected cell, next to the rhizoid, died, increased accumulation of FD was observed in the growing rhizoidal cell in 48 h (Fig. 2E & F). In most case, 3 kDa FD was detected in all cells in the filament a week after injection especially around pit plugs (Fig. 2G & H). No cytoplasmic streaming has not been observed in G. monilis cells. The injected plants could survive, with no visible damage for several weeks, if not subjected to excessive fluorescent light.
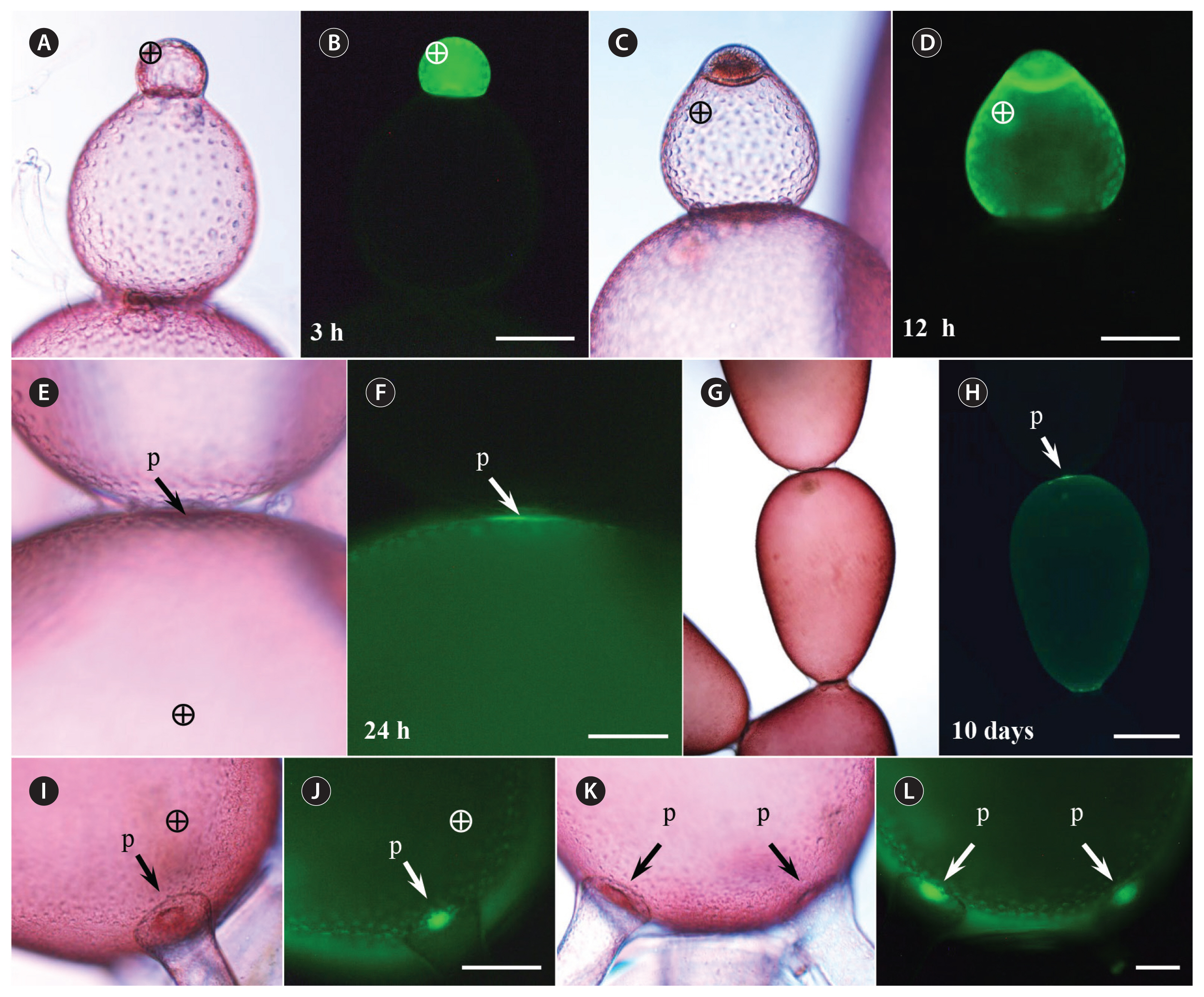
When injected with 10 and 20 kDa FD, intracellular diffusion as well as intercellular transport occurred much more slowly than with 3 kDa FD (Fig. 3). Fluorescent probes diffused slowly and remained around the injected site 30 min after injection (Fig. 3A & B). Migration of 10 kDa FD to adjacent cells was not observed until 3 h after injection, but adjacent pit-connections became brighter than other parts of the cell (Fig. 3C & D). Even when the probe was injected into small apical cells, diffusion progressed slowly (Fig. 3E & F). Migration of 10 kDa FD to the adjacent cells was observed within 24 h after injection (Fig. 3G & H). The probes transferred to 5–6 cells from the injected cell in a week (Fig. 3I & J), but a large fraction of 10 kDa FD remained in the injected cell 1 week after injection (Fig. 3K & L). A small portion of the injected 20 kDa FD was transported to an adjacent cell within 24 h (Fig. 3M–P). Transport ceased in the next cells, and no further migration to subsequent cells, was observed 1 week after injection.
Fluorescent probes above 40 kDa were not transported to the adjacent cells (Fig. 4). Although the probes spread evenly in the injected cell within 3 h (Fig. 4A & B), no transfer to the neighboring cells was observed, except when the injected cell divided (Fig. 4C & D). The fluorescent probes began to appear near pit-connections 24 h after injection (Fig. 4E & F), but the probes were not transmitted further and remained in the injected cell up to 10 days after injection (Fig. 4G & H). When a regenerating rhizoid cell was induced to form by cutting off the basal cell from the injected cell, strong accumulation of the fluorescent probes was observed near pit-connections within 48 h after injection (Fig. 4J & L).
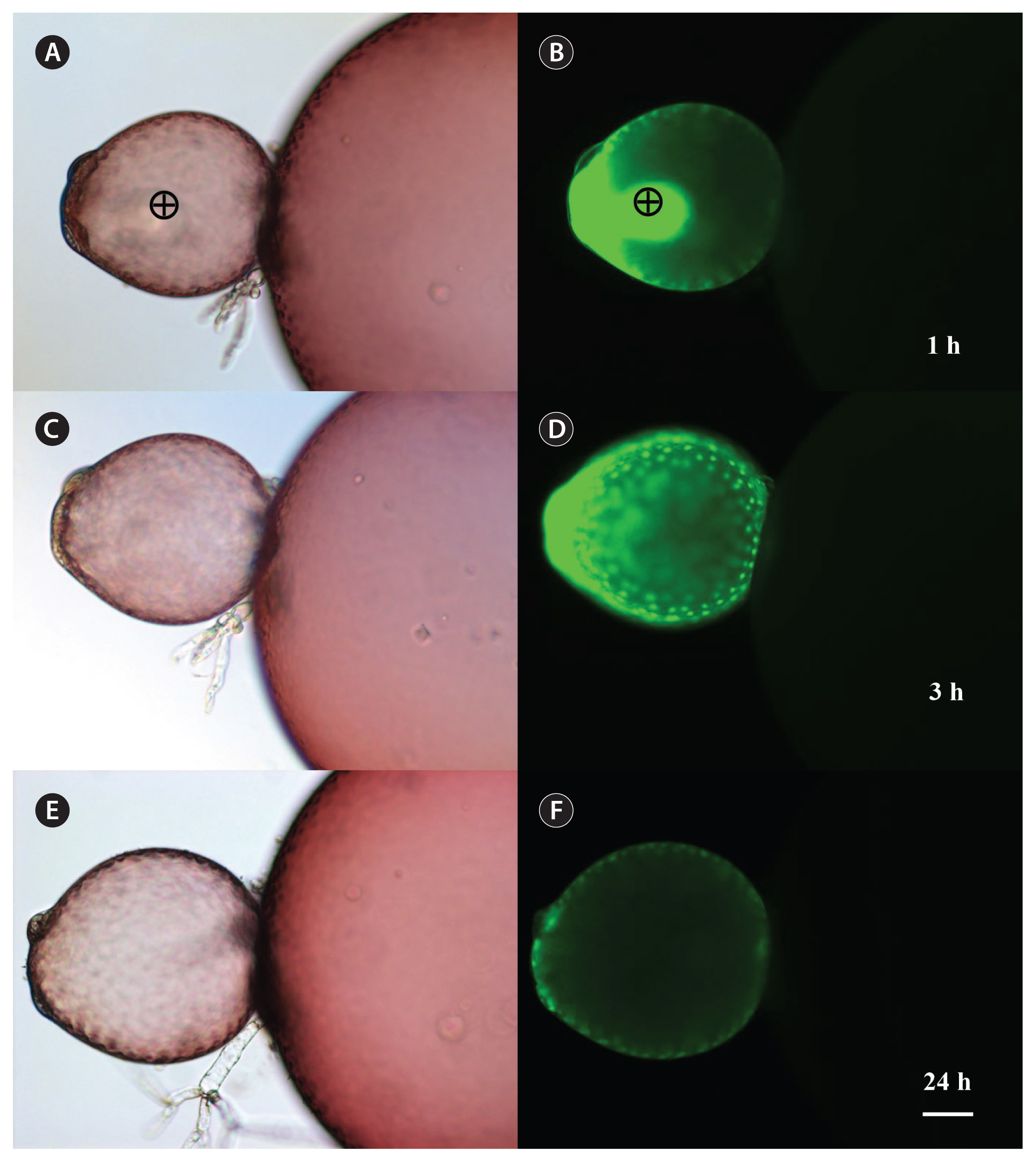
Recombinant green fluorescence protein was injected into the apical cells and intercellular translocation was observed (Fig. 5). The molecular weight of rGFP was almost 30 kDa on sodium dodecyl sulfate polyacrylamide gel electrophoresis (Nagasato et al. 2017). rGFP spread in the injected cell within 3 h similar to FD (Fig. 5D). Migration of rGFP to the neighboring cells was not observed 24 h after injection and most fluorescence disappeared in the injected cell except some fluorescence near pit-connections (Fig. 5F).
DISCUSSION
Our results showed that pit-connections are conduit for molecules between adjacent cells and that these conduits allow intercellular communication in multicellular red algae. The function of pit-connections is key to understanding the successful evolution of multicellularity in red algae. The pit-connection is the only place where two adjacent cells are interconnected. Ultrastructural studies showed that the pit plug, tightly fitting in the center of the pit-connection, appears to be a formidable barrier for the intercellular transport of any macromolecules (Pueschel 1987, 1990), but their appearance is not evidence that it doesn’t occur. Pit-connections have long been suspected to be involved in the transfer of materials between cells (Wetherbee 1979), particularly between colorless red algal parasites and their photosynthetic red algal hosts, connected only by secondary pit-connections (i.e., Wetherbee and Quirk 1982, Goff et al. 1996). However, the structural diversity of pit plugs, membranes as well as caps on some pit plugs, complicates the notion of the function of pit-connection as conduits of transport. It has been generally assumed that, if there is any intercellular transports in red algae, only ions, small signalling chemicals, or lipophilic molecules, pass around the pit plug to the next cell (Koslowsky and Waaland 1987). Our results showed that relatively large (up to 20 kD) macromolecules pass between cells, probably through the gap between the pit plug and the plasmalemma that is continuous between cells along the sides of the pit plug. The difference in intracellular pressure between two neighboring cells could facilitate the movement of substances through this gap. In dividing and expanding cells of G. monilis, a difference in intercellular pressure could be generated between expanded and expanding cells. Fluorescent tracers were transported faster towards the growing tips and reproductive cells, supporting this hypothesis. The full mechanism of this transport and the movement past the pit plugs needs further elucidation.
Our results suggested that the movement of macromolecules within the cytoplasm or through the pit plug may not be simply due to diffusion. Red algae do not exhibit the rapid cytoplasmic streaming observed in cells of many other multicellular organisms (Goff and Coleman 1987, Pueschel 1990). Due to the physical size of the injected cells, being on the order of 100–300 μm and in the absence of detectable cytoplasmic streaming in G. monilis cells the times involved for intracellular diffusion of F-dextran and rGFP molecules would be long. If the movement of molecules is solely by diffusion a signal gradient would be predicted, with a high intensity at the microinjection sites falling to a weaker signal at the opposite sides of the cell. However, we observed that tracer spread in the injected cell fast and tracer signals accumulated unevenly in the cytoplasm, for example adjacent to the pit-connection and in newly forming cells. Such patterns suggest the presence of a more complex process than simple diffusion-based movement of the test probe. Previous freeze-etch study on the ultrastructure of pit plugs showed membranous particles that could represent transport proteins (Pueschel 1977). Future microinjection studies using cytoskeletal inhibitors may provide hints for this transport system.
The size exclusion limit of pit-connection was comparable to that of plasmodesmata reported in plants. Our results showed that fluorescent tracers with size over 20 kDa can be transported among adjacent cells. Pit-connections in red algae appear to be a transport system analogous to those in plants and animals, and can aid in cellular communication and coordination in multicellular red algae. Further study is necessary to elucidate the mechanism by which pit-connections regulate intercellular transport between normal vegetative cells and how regulation might be modified during the differentiation of specialized reproductive cells.