ABSTRACT
Sargassum macrocarpum is a rich source of anti-inflammatory compounds. Recently, one of the compounds, tuberatolide B, has been reported as a functional anti-inflammatory additive for foods and nutraceuticals. The artificial seeding, growth and maturation of S. macrocarpum were investigated from May 2018 to September 2019. Indoor culture experiments for induction of egg release were conducted at temperatures of 17, 20, 23, and 26°C and irradiances of 0, 10, 20, 40, and 80 μmol photons m−2 s−1 under 14 : 10 h (L : D) photoperiod. Within a given treatment combination, higher temperatures and irradiance levels favoured the maturation of receptacles in S. macrocarpum. Using artificial temperature and irradiance control, thalli matured one month earlier than thalli in nature. Under natural condition, receptacle formation began in April, and the eggs were released in June and July. The release of eggs from the receptacles was promoted at 17–20°C and 40–80 μmol photons m−2 s−1, and the fastest growth of germlings occuring at 15–17°C and 40 μmol photons m−2 s−1. For mature thalli, 300 g wet-weight was sufficient to seed 100 m of seed string. Thalli grew to 10.5 ± 2.6 cm in length at a density of 6.7 ± 3.3 individuals m−1 after 1 year of cultivation, from germination. This study demonstrates that it is possible to cultivate S. macrocarpum for the production of anti-inflammatory products.
INTRODUCTIONThere are 30 species of Sargassum species reported in Korea (Oak and Lee 2006). The perennial Sargassum macrocarpum C. Agardh has a wide distribution in Japan (Murase et al. 2000) and Korea (Oak and Lee 2006). This alga usually grows at depths of 10 m or more, forming dense stands contributing to the sea forest. The Sargassum sea forests play important ecological roles in the coastal ecosystem (Murase and Kito 1998) due to their large biomass and high productivity. These beds provide nursery areas to commercially important fish species and help to preserve environmental conditions (Yoshida et al. 1963, Murase et al. 2000). Surprisingly, Sargassum rafts act as a substratum for numerous epibiotic organisms, providing them with habitat, a food source and a mode of dispersal (Kim et al. 2019b, Kwon et al. 2019). Therefore, considerable information has been accumulated on their growth, maturation period and cultivation techniques from ecological and industrial viewpoints.
S. macrocarpum are not ideal canidiates for aquaclture as their solid conical holdfast (Ko et al. 2019), is believed to less well suited to regeneration of new fronds from the holdfast than S. fulvellum or S. fusiforme which have dendritic or fibrous holdfasts (Oak and Lee 2006). However, it has been reported that an anti-inflammatory substance (Kim et al. 2019a) can be extracted from the alga, and consequently, there is renewed interest in developing aquaculture techniques for this species.
Previous studies have shown that Sargassum species contain terpenoids, polysaccharides, polyphenols, sargachromenol, steroids, and plastoquinones (Yende et al. 2014), which possess anti-oxidant (Kim et al. 2007), anticholine esterase inhibitory (Choi et al. 2007), anti-cancer (Zandi et al. 2010), anti-inflammatory (Kang et al. 2008, Sanjeewa et al. 2019), immunomodulatory (Chandraraj et al. 2010), and other biological activities (Kim et al. 2018). Compounds extracted from S. macrocarpum have been shown to inhibit the CpG-induced inflammatory response in bone marrow-derived macrophages and derived dendritic cells (Kim et al. 2019a). Inflammation has become one of the leading causes of morbidity worldwide because of the overproduction inflammatory mediators in many serious diseases such as arthritis, asthma, vascular disease, dermatitis, migraines, obesity, and other diseases (Islam et al. 2013, Fernando et al. 2016, Kim et al. 2016, 2019a).
Despite the economic value of Sargassum, S. fulvellum is currently the only artificially cultivated Sargassum species, being typically for human consumption and sea reforestation. Since the demand for S. macrocarpum is likely to increase in the future, S. macrocarpum has potential commercial cultivation in Korea. In order to protect the natural resource from overharvesting, it is important to develop mariculture techniques for this species. This paper reports on studies on the artificial seeding, growth, maturation, and culture conditions for the commercial cultivation of S. macrocarpum.
MATERIALS AND METHODSSample collecting
S. macrocarpum plants were collected monthly from the rocky areas in 3–5 m depth at Jocheon (33°32′23″ N, 126°37′44″ E), Jeju Island, Korea from May 2018 to April 2019. During each collection, three 1 × 1 m quadrats were randomly placed on the benthos, and all S. macrocarpum thalli inside the quadrats were collected and transported to the laboratory. Once in the laboratory, thalli were cleaned of epiphytes and rinsed with filtered seawater. All the thalli were measured and weighed. The seawater temperature at the sampling site was measured using a Hobo UA-002-64 data logger (Onset, Bourne, MA, USA).
Induction of egg releaseIndoor culture experiments were undertaken in June 2018. Reproductive plants were transported to the laboratory immediately after collection, were rinsed in sterile, filtered seawater, and the receptacles were excised. The receptacles were immersed in 1% Betadine solution for a few seconds and then incubated at 20 ± 0.5°C in an antibiotic mixture solution (Guillard 1968) for one day. After being cleaned, the receptacles were cultured in Petri dishes (10 explants in each of triplicate) with 20 mL of PESI culture medium. Egg release from the receptacles was measured at temperatures of 17, 20, 23, and 26°C under 40 μmol photons m−2 s−1 and 14 : 10 h (L : D), and irradiances of 0, 10 20, 40, and 80 μmol photons m−2 s−1 under 20°C and 14 : 10 h (L : D). Irradiance was measured at the surface on the sterilized Petri dishes using a LI-1500 Data logger (Li-Cor, Lincoln, NE, USA). In all the cultures, low temperature incubators (HB-103S; HanBaek Scientific Co., Bucheon, Korea) were used to control the photoperiod at 14 : 10 h (L : D). Egg release was determined as the percentage of explants in each treatment showing egg release, under microscopic observation (n = 10 in each of triplicate).
Germling growthMature thalli were moved to plastic dishes (50 cm diameter, 20 cm depth), and an embryo solution was created by rubbing the mature thalli which had embryos in their receptacles. The liberated embryos that sank onto the bottom of the plastic dishes were collected in a mesh net (ca. 300–500 μm in mesh size) and washed several times with freshly filtered seawater. After being cleaned, the embryos were cultured in triplicate Petri dishes with 20 mL of PESI culture medium. Culture conditions for germling growth were 40 μmol m−2 s−1 and 14 : 10 h (L : D) for temperature experiment (5, 10, 15, 20, and 25°C), 20°C and 14 : 10 h (L : D) for irradiance experiment (5, 10, 20, 40, and 80 μmol photons m−2 s−1), 20°C and 40 μmol photons m−2 s−1 for photoperiod experiment (10 : 14, 12 : 12, 10 : 14 h, L : D) at the surface on the sterilized Petri dishes, were controlled by EYELA incubators (MTI-202B; Tokyo, Japan). Growth was measured as the length of each whole thallus using a microscope (CKK41; Olympus, Tokyo, Japan) with a digital CCD camera (DP27; Olympus). The culture experiments lasted for 30 days, and the initial and final length of thalli was measured. The relative growth rate (RGR) was calculated by the following formula (Serisawa et al. 2002, Hwang et al. 2018):
, where Va is the value of the variable (length) at time t2, Vb is the value at time t1, and t is the number of days from t1 to t2.
Artificial seedingAs a seeding material for the embryos, PVC frames (ca. 35 cm height × 45 cm width), holding a total length of 100 m of seed string made of mixed nylon and polypropylene fibres were used for Sargassum cultivation. The collected embryos in suspension were panted on to the seed frames with a paintbrush. Seedlings of S. macrocarpum were reared in an indoor tank for 90 days from June to September 2018 until they were up to 10–15 mm in length. The tank used for the seedling culture was 1 m wide, 10 m long and 60 cm deep. Fresh seawater and air were continuously supplied, through a pipe, to the tank. Water temperature was not controlled. Illumination was regulated using shade cloth, to about 64.4–96.6 μmol photons m−2 s−1 (the readings were taken on the water surface, at noon each day). During the seeding and culture, the length and number of primary thalli on the seed string and the number of laterals were measured once a week.
Nursery and main cultivationAfter three months of tank culture, seedlings were transferred to the nursery farm in Hwabuk, Jeju Island where they were held from September to November 2018. Hwabuk is 6 km from Jocheon and has an environment similar to Jocheon. It is an area where artificial reefs are installed at a depth of 3–6 m, making it a convenient place to establish the trial in a sheltered environment. The seedlings were transferred to the main cultivation farm where they were on-grown from November 2018 to September 2019 using a long-line system, described by Hwang et al. (2006). The seed strings were attached to a 100 m culture line (3 mm diameter, 50 mm length) every 10 cm. The main culture line was held at 3 m depth, using plastic buoys. Culture ropes were periodically cleaned of fouling. Biological variables such as length of thalli and biomass per culture rope were measured monthly during the culture period.
Statistical analysisData were analyzed with one-way ANOVA. Homogeneity of variances was verified using the Levene’s test. When the ANOVA revealed significant differences (p < 0.05), a post hoc Tukey’s honest significant difference test was applied. Data were analyzed using SPSS ver. 8.0 and SYSTAT ver. 9.0 (SPSS Inc., Chicago, IL, USA).
RESULTSGrowth and maturationSeawater temperature in the natural habitat of S. macrocarpum varied from 12.7 to 29.4°C during the experiment (Fig. 1). The maximum seawater temperature was recorded in August 2018, and the minimum in February 2019. S. macrocarpum started to grow when the seawater temperature decreased below 20°C in October. Thalli reached a mean maximum length of 135.3 ± 3.2 cm in June and a mean minimum length of 53.1 ± 2.7 cm in October. After October, the growth of thalli started to increase. In nature, receptacle formation was observed from April to July when seawater temperature was 16.1–22.9°C. The peak period for egg release from female receptacles was from June to July.
Egg release and germling growthObservations of mature thalli (Fig. 2A) indicated that egg release from the receptacles occurred in culture (Fig. 2B) for both male and female receptacles (Fig. 2C–H). The embryos (Fig. 2I) started to grow rhizoids after 2 days, and the germlings formed an early blade (Fig. 2J) after 10 days later. The second, third and fourth blades formed after 25, 42, and 56 days, respectively (Fig. 2K–M). Temperature and irradiance significantly affected egg release (one-way ANOVA, p < 0.01) (Fig. 3A & B). After 9 days of culture under 17°C and 40 μmol photons m−2 s−1, the egg release rate reached a maximum value of 89.5%.
For the 30 days of indoor culture, the young thalli had responded differently to the different temperature and photoperiod conditions (Fig. 4). The RGR of thalli was the highest at 15°C, reaching a maximum value of 0.9 ± 0.1% d−1 (one-way ANOVA, p < 0.01) (Fig. 4A). The RGR of thalli was not significantly different between 5 and 40 μmol photons m−2 s−1 but significantly decreased under higher irradiance conditions (one-way ANOVA, p < 0.05) (Fig. 4B). The RGR of thalli was the highest at 12 : 12 h (L : D) and the lowest at 10 : 14 h (L : D) (one-way ANOVA, p > 0.05) (Fig. 4C).
Nursery and main cultivationThe seed frames with attached germlings were transferred to the nursery culture ground from September to November 2018 (Fig. 5). After 2 months of nursery culture, germlings grew to a mean length of 4.2 ± 0.3 mm with a relatively narrow range of between 3 and 5 mm (Table 1). The density of the germlings varied between 2.2 and 15.4 per centimetre of seed string (Table 1). Epiphytic algae, copepods and hydrozoans were observed on the seed strings during the nursery culture phase. During the main cultivation, from November 2018 to September 2019, young thalli grew to 10.5 ± 0.6 cm in length (Table 2) and the density of the young thalli varied between 6.7 and 25.2 plants per meter of culture rope (Table 2).
DISCUSSIONNatural products and their derivatives have been recognized for many years as a source of therapeutic agents that have a wide range of multidimensional chemical structures (Sircar 1982). The marine environment is a source of structurally unique secondary metabolites produced by different organisms such as sponges, tunicates, bryozoans, soft corals, molluscs, microorganisms and seaweeds (Blunt et al. 2011). Seaweed exploitation accounts for a market of over US$ 6 billion dollars per year (Food and Agriculture Organization of the United Nations 2003, Smit 2004), with a total annual use estimated at 8 million tons. Seaweeds are the most abundant source of polysaccharides, including alginates, agar and carrageenan (Laurienzo 2010), however, the development of seaweed secondary metabolites as therapeutic or antifouling agents is still in its infancy. The common uses are related to the food and cosmetic industries; however, biotechnological applications are rapidly expanding and hydrogels currently account for 10% of this market (Laurienzo 2010).
Due to the high costs and potentially adverse side effects associated with anti-inflammatory drugs as long-term treatments, screening of natural sources of anti-inflammatory compounds with minimal side effects has drawn much attention (Oh et al. 2016). S. macrocarpum extracts have been shown to have anti-inflammatory properties through a wide range of activity, These include inhibition of nitric oxide and prostaglandin E2 production and inhibition of the expression of inducible nitric oxide synthase and cyclooxygenase-2 at the mRNA and protein levels. Moreover, a strong inhibitory effects on the production of interleukin (IL)-12 p40, IL-6, and tumour necrosis factor α in CpG-stimulated bone marrow-derived macrophage and bone marrow-dendritic cell (Manzoor et al. 2014) have been shown to have anti-inflammatory effect on bone marrow-derived macrophages and dendritic cells (Cheon et al. 2017).
Seaweed mariculture generally results in less environmental impact and degradation than the harvesting of wild populations (Kapraun 1999). The present research shows that mass production of embryos of S. macrocarpum is possible, with high germling survival observed in the indoor culture trials (Table 1). The initial survival rate of germling was 30–40% when cultured indoors in the currently industrially produced S. fulvellum and S. fusiforme (Hwang 1997, Hwang et al. 2006), which was similar to that of the S. macrocarpum in this study. It can be said that this suggests that there is a high possibility of stable artificial seedling production of S. macrocarpum. This technology will permit the cultivation of a seedstock of germlings for aquaculture and sea forest reforestation.
The onset of regeneration of S. macrocarpum occurs at the end of the growth period in October. Formation of reproductive branch started in February, receptacle formation began in April, and embryo release continued until July (Fig. 1). The pattern of growth and maturation of S. macrocarpum showed similar pattern to S. fusiforme (Hwang 1997). Reproductive thalli can be induced the release of eggs in the laboratory, and the optimal conditions for release occurred when receptacles were maintained at 17°C and 80 μmol photons m−2 s−1 (Fig. 2). Significant mortalities occurred when thalli were held at temperatures in excess of 23°C. Egg release rates also increased at higher irradiance under the appropriate temperature conditions.
In this study, growth of S. macrocarpum from Jeju island germlings was maximized at 15°C, 40 μmol photons m−2 s−1 and 12 : 12 h (L : D) (Fig. 4). In contrast, Yoshida et al. (1997) reported that the growth of germlings of S. macrocarpum from Hiroshima Bay, Japan was maximized at 15°C and 100 μmol photons m−2 s−1. The tolerance limits and optimum temperature may also vary within the geographic range of a species, as a function of genotypic adaptation, resulting in the presence of distinct “eco-types” with different tolerance limits and optimum temperatures (Davison 1991, Boderskov et al. 2016).
S. macrocarpum exhibits significant differences when compared to S. fulvellum and S. horneri during the seed production phase. S. fulvellum and S. horneri release a large amount of mucus along with the embryos (Uchida 1993, Hwang et al. 2005), which helps the eggs to attach to the spore strings. S. macrocarpum produces less mucus resulting in a lot of embryo’s shedding and a lower germling density. S. fulvellum and S. horneri, the harvestable biomass is reached within 1 year from seed production to main cultivation, whereas in S. macrocarpum harvestable biomass is reached after 2 years. Slow growth rate may be problematic in terms of the economic viability of mass cultivation of these seaweeds. During the nursery and main cultivation trials, there was no grazing loss by herbivorous fishes but the densities of plants decreased (Tables 1 & 2) due to physical disturbances such as wave action associated with typhoons.
The slow growth rate of S. macrocarpum poses challenges for wild harvest and commercial aquaculture. In the wild S. macrocarpum grows slowly for one year but begins to grow rapidly and becomes mature after two years with a lifespan of more than nine years (Murase and Kito 1998). Under culture conditions, Murase (2001) found that the growth of juvenile thalli was also very slow, reaching only 10 cm or less in total length after approximately one year from germination and reaching about 15 cm in total length after 1.5 year growth. Similarly, in this study, S. macrocarpum reached a total length of 10 cm within 1 year after germination. In case of Sargassum genus that breeds in embryos like S. fulvellum and S. fusiforme, the growth of thalli due to germling is very slow within 10 cm in the first year, and in the second year reproduction it grows rapidly to 50 cm−1 m (Hwang 1997, Hwang et al. 2006). It is expected that the S. macrocarpum will show a similar trend, for which additional outdoor culture experiments are needed.
The artificial propagation of S. macrocarpum will enable this species to be used in an important ecological role to create seaweed forests in Jeju Island, Korea and will also increase the potential for industrial utilization of this species. However, due to the long grow-out periods for S. macrocarpum compared to other species, commercial cultivation will require greater investment and incur higher opportunity costs than other farmed seaweed species.
ACKNOWLEDGEMENTSThis work was supported by a grant from the National Institute of Fisheries Science (R2020004, P2020044). The authors would like to thank Dr. Philip Heath (Tisbe Ltd., New Zealand) for reviewing the English.
Fig. 1Fluctuations in mean length of Sargassum macrocarpum C. Agardh and seawater temperature at 10 m depth at Jocheon, Jeju Island, Korea from May 2018 to April 2019. Gray area indicates a maturation period in nature and the vertical bars represent the standard deviation. 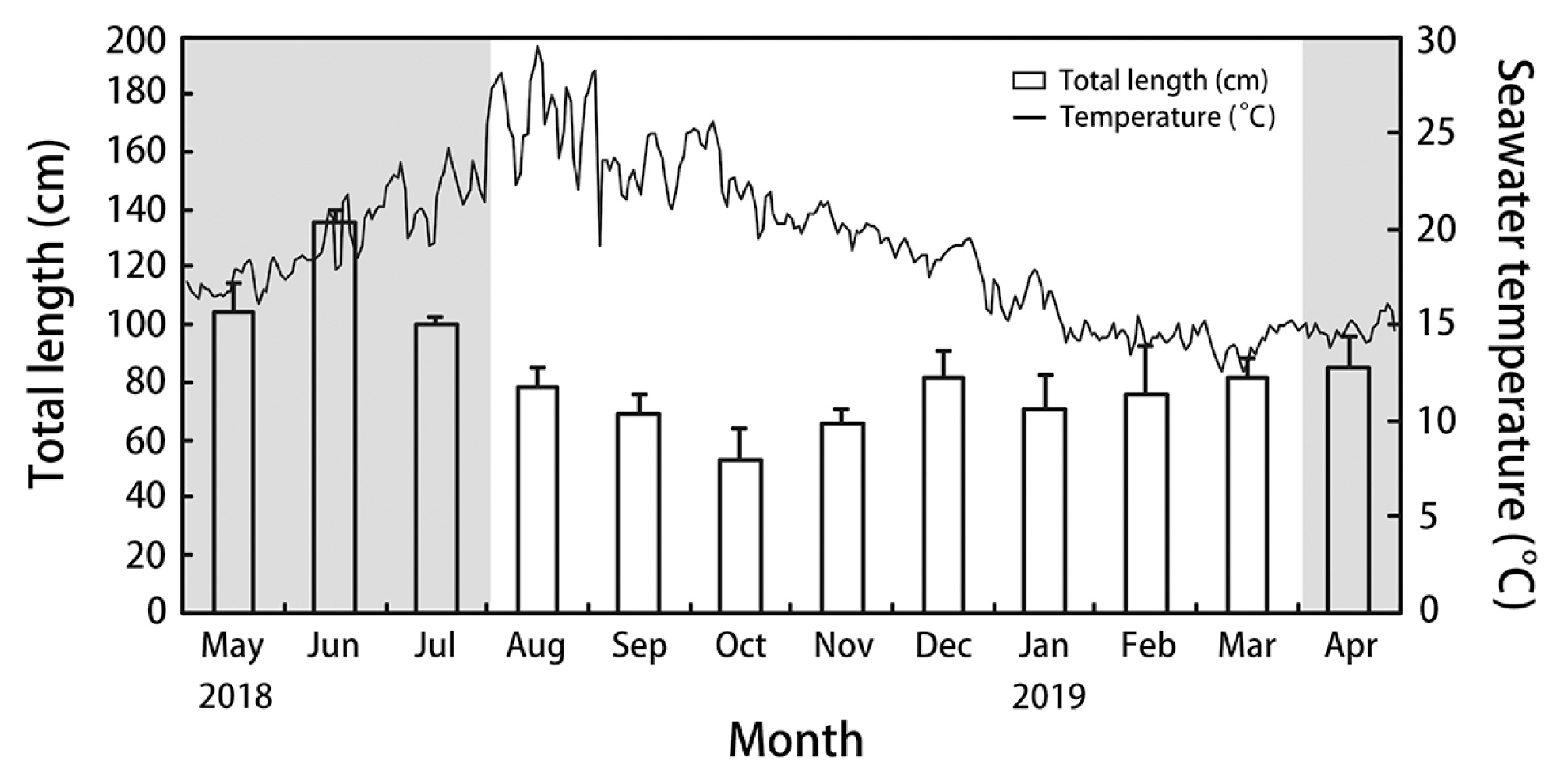
Fig. 2Early development of Sargassum macrocarpum C. Agardh. (A) Thalli. (B) Matured receptacles (arrowheads). (C) A female branch. (D) A male branch. (E) A female receptacle. (F) A male receptacle. (G) Cross-sectioned view of the female receptacle. (H) Cross-sectioned view of the male receptacle. (I) Released embryos. (J) A germling after 10 days culture. (K) A germling after 25 days culture. (L) A germling after 42 days culture. (M) A germling after 56 days culture under 15°C, 20 μmol m−2 s−1, 10 : 14 L : D. Scale bars represent: A, 10 cm; B–F, 1 cm; G–I, 200 μm; J–M, 500 μm. 
Fig. 3Effect of temperature and irradiance on egg release from receptacles of Sargassum macrocarpum C. Agardh during 10 days of cultuse. Culture conditions were 40 μmol m−2 s−1 and 14 : 10 h (L : D) for temperature experiment (A), and 20°C and 14 : 10 h (L : D) for irradiance experiment (B). Values are presented as mean ± standard error (n = 30). 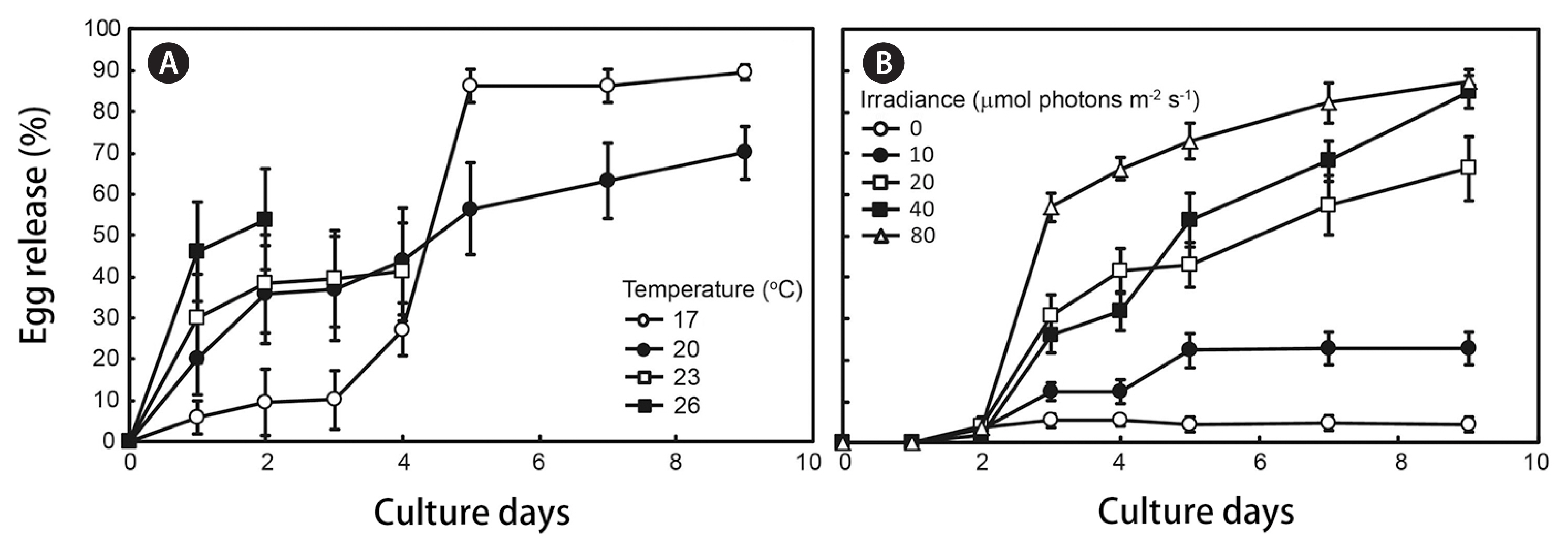
Fig. 4Effect of temperature, irradiance and photoperiod on early growth of germlings of Sargassum macrocarpum C. Agardh after 30 days culture. Culture conditions were 40 μmol m−2 s−1 and 14 : 10 h (L : D) for temperature experiment (A), 20°C and 14 : 10 h (L : D) for irradiance experiment (B), 20°C and 40 μmol m−2 s−1 for photoperiod experiment (C). Values are presented as mean ± standard error (n = 60). 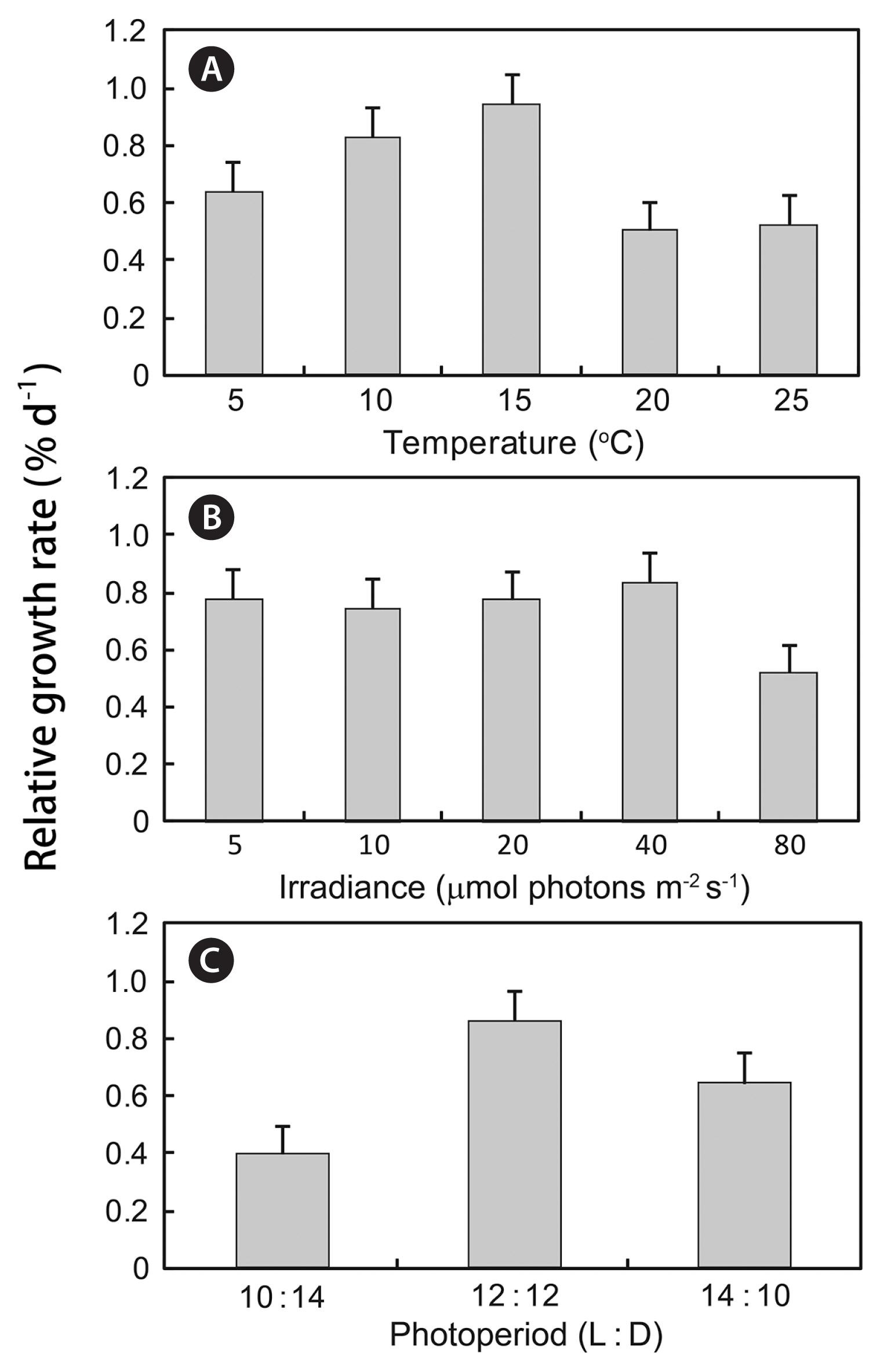
Fig. 5Artificial seeding, nursery and main culture process of Sargassum macrocarpum C. Agardh. (A) Mature thalli. (B) Eggs attached on receptacles. (C) Released embryos. (D) Dense embryo suspension and seeding of the embryos on a seed frame by a paintbrush (frame is 45 cm × 35 cm, holding a total length of 100 m of string made of mixed nylon and polypropylene fibres). (E) Tank culture of seed frames after seeding. (F) Nursery culture of seed frames at 3 m depth. (G) Young thalli attached on seed frames. (H) Young thalli growing on main culture rope at July 2019. Scale bars represent: B, 1 cm; C, 200 μm. 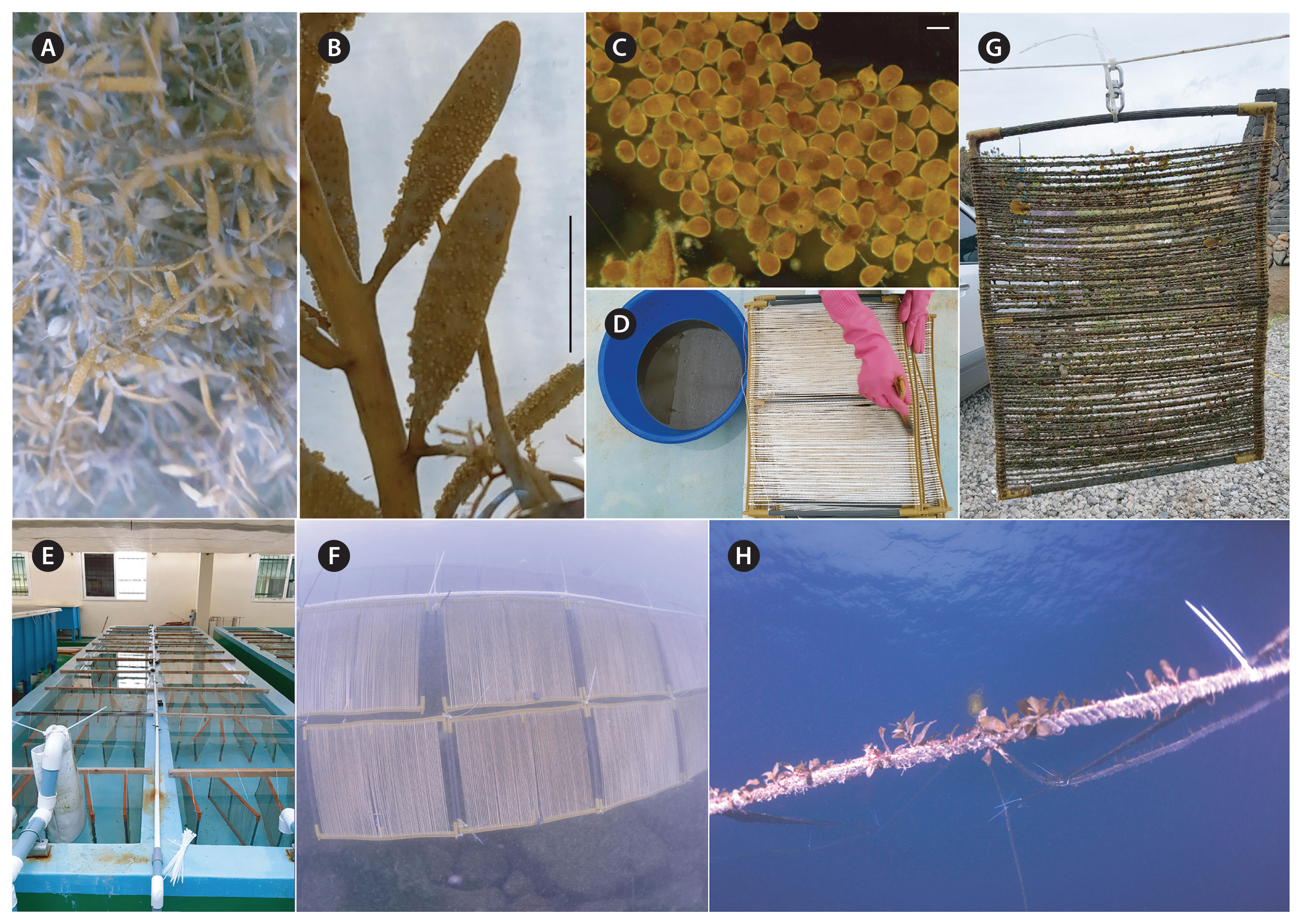
Table 1Environments and growth of germlings of Sargassum macrocarpum C. Agardh during nursery culture from September to November 2018 at Hwabuk, Jeju Island, Korea
Table 2Environments and growth of Sargassum macrocarpum C. Agardh during the main cultivation from November 2018 to September 2019 at Hwabuk, Jeju Island, Korea
c Destroyed after 7 typhoons from July to September 2019 (Korea Meteorological Administration 2019). REFERENCESBlunt, JW., Copp, BR., Munro, MHG., Northcote, PT. & Prinsep, MR. 2011. Marine natural products. Nat Prod Rep. 28:196–268.
Boderskov, T., Schmedes, PS., Bruhn, A., Rasmussen, MB., Nielsen, MM. & Pedersen, MF. 2016. The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) growth in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. J Appl Phycol. 28:1153–1165.
Chandraraj, S., Prakash, B. & Navanath, K. 2010. Immunomodulatory activities of ethyl acetate extracts of two marine sponges Gelliodes fibrosa and Tedania anhelans and brown algae Sargassum ilicifolium with reference to phagocytosis. Res J Pharma Biol Chem Sci. 1:302–307.
Cheon, JM., Kim, HS., Choi, EO., Kwon, DH., Choi, YH., Kim, BW. & Hwang, HJ. 2017. Anti-inflammatory activities of an ethanol extract of Sargassum macrocarpum in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. J Life Sci. 27:1437–1444.
Choi, BW., Ryu, GS., Park, SH., Kim, ES., Shin, JH., Roh, SS., Shin, HC. & Lee, BH. 2007. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for Alzheimer’s disease therapy. Phytother Res. 21:423–426.
Fernando, IPS., Nah, J. & Jeon, Y-J. 2016. Potential anti-inflammatory natural products from marine algae. Environ Toxicol Pharmacol. 48:22–30.
Food and Agriculture Organization of the United Nations. 2003. Review of the state of world aquaculture. FAO Fisheries Circular. Food and Agriculture Organization of the United Nations, Rome, 95 pp.
Guillard, RRL. 1968. A simplified antibiotic treatment for obtaining axenic cultures of marine phytoplankton. Mimeographed document. Woods Hole Oceanography Institute of Marine Biology Laboratory, Woods Hole, MA, 9 pp.
Hwang, EK. 1997. Artificial seed production using the reproduction methods in Hizikia fusiformis (Phaeophyta). PhD dissertation. Pukyong National University, Busan, 139 pp.
Hwang, EK., Baek, JM. & Park, CS. 2005. Growth, maturation and development of Sargassum fulvellum (Sargassaceae, Phaeophyta). J Korean Fish Soc. 38:112–117.
Hwang, EK., Ha, DS. & Park, CS. 2018. The influences of temperature and irradiance on thallus length of Saccharina japonica (Phaeophyta) during the early stages of cultivation. J Appl Phycol. 30:2875–2882.
Hwang, EK., Park, CS. & Baek, JM. 2006. Artificial seed production and cultivation of the edible brown alga, Sargassum fulvellum (Turner) C. Agardh: developing a new species for seaweed cultivation in Korea. J Appl Phycol. 18:251–257.
Islam, MN., Ishita, IJ., Jin, SE., Choi, RJ., Lee, CM., Kim, YS., Jung, HA. & Choi, JS. 2013. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents phelphorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem Toxicol. 55:541–548.
Kang, JY., Khan, MNA., Park, NH., Cho, JY., Lee, MC., Fujii, H. & Hong, YK. 2008. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol. 116:187–190.
Kapraun, DF. 1999. Red algae polysaccharide industry: economics and research status at the turn of the century. Hydrobiologia. 398/399:7–14.
Kim, EA., Kim, SY., Kim, J., Oh, JY., Kim, HS., Yoon, WJ., Kang, D-H. & Heo, S-J. 2019a. Tuberatolide B isolated from Sargassum macrocarpum inhibited LPS-stimulated inflammatory response via MAPKs and NF-κB signaling pathway in RAW264.7 cells and zebrafish model. J Funct Foods. 52:109–115.
Kim, HM., Jo, J., Park, C., Choi, BJ., Lee, H-G. & Kim, KY. 2019b. Epibionts associated with floating Sargassum horneri in the Korea Strait. Algae. 34:303–313.
Kim, H., Sanjeewa, KKA., Fernando, IPS., Ryu, B., Yang, H., Ahn, G., Kang, MC., Heo, S., Je, J. & Jeon, Y-J. 2018. A comparative study of Sargassum horneri Korea and China strains collected along the coast of Jeju Island South Korea: its components and bioactive properties. Algae. 33:341–349.
Kim, K., Ko, S., Ye, B., Kim, M., Kim, J., Ko, E., Cho, H., Kim, D., Heo, S. & Jung, W-K. 2016. 5-Bromo-2-hydroxy-4-methyl-benzaldehyde inhibited LPS-induced production of pro-inflammatory mediators through the inactivation of ERK, p38, and NF-κB pathways in RAW 264.7 macrophages. Chem Biol Interact. 258:108–114.
Kim, S., Choi, D., Athukorala, Y., Jeon, Y., Senevirathne, M. & Rha, CK. 2007. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum
. J Food Sci Nutr. 12:65–73.
Ko, SJ., Kim, YK., Hong, SW., Kang, MS., Hwang, EK. & Lee, YD. 2019. Application of reproductive allocation index to the analysis of growth and maturation pattern of Sargassum macrocarpum C. Agardh in Jeju Island, Korea. Korean J Environ Biol. 37:672–681.
Korea Meteorological Administration. 2019. Weather information (Typoon), Available from: http://www.weather.go.kr/weather/typoon/report.jsp
. Accessed Dec 30, 2019
Kwon, K., Choi, BJ., Kim, KY. & Kim, K. 2019. Tracing the trajectory of pelagic Sargassum using satellite monitoring and Lagrangian transport simulations in the East China Sea and Yellow Sea. Algae. 34:315–326.
Laurienzo, P. 2010. Marine polysaccharides in pharmaceutical applications: an overview. Mar Drugs. 8:2435–2465.
Manzoor, Z., Mathema, VB., Chae, D., Yoo, E., Kang, H., Hyun, J., Lee, NH., Ko, M. & Koh, Y-S. 2014. Extracts of the Seaweed Sargassum macrocarpum inhibit the CpG-induced inflammatory response by attenuating the NF-κB pathway. Food Sci Biotechnol. 23:293–297.
Murase, N. 2001. Ecological study of Sargassum macrocarpum C. Agardh (Fucales, Phaeophyta). J Shimonoseki Univ Fish. 49:131–212.
Murase, N. & Kito, H. 1998. Growth and maturation of Sargassum macrocarpum C. Agardh in Fukawa Bay, the Sea of Japan. Fish Sci. 64:393–396.
Murase, N., Kito, H., Mizukami, Y. & Maegawa, M. 2000. Productivity of a Sargassum macrocarpum (Fucales, Phaeophyta) population in Fukawa Bay, Sea of Japan. Fish Sci. 66:270–277.
Oak, JH. & Lee, IK. 2006. Taxonomy of the genus Sargassum (Fucales, Phaeophyceae) from Korea II. Subgenus Bactrophycus section Halochloa and Repentia
. Algae. 21:393–405.
Oh, J., Kim, J. & Lee, Y. 2016. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr Res Pract. 10:42–48.
Sanjeewa, KKA., Fernando, IPS., Kim, S., Kim, W., Ahn, G., Jee, Y. & Jeon, Y-J. 2019.
Ecklonia cava (Laminariales) and Sargassum horneri (Fucales) synergistically inhibit the lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK pathways. Algae. 34:45–56.
Serisawa, Y., Yokohama, Y., Aruga, Y. & Tanaka, J. 2002. Growth of Ecklonia cava (Laminariales, Phaeophyta) sporophytes transplanted to a locality with different temperature conditions. Phycol Res. 50:201–207.
Sircar, NN. 1982. Medicinal plants. East Pharm. 29:49–52.
Smit, AJ. 2004. Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol. 16:245–262.
Uchida, T. 1993. The life cycle of Sargassum horneri (Phaeophyta) in laboratory culture. J Phycol. 29:231–235.
Yende, SR., Harle, UN. & Chaugule, BB. 2014. Therapeutic potential and health benefits of Sargassum species. Pharmacogn Rev. 8:1–7.
Yoshida, G., Arai, S. & Terawaki, T. 1997. Effects of irradiance and temperature on the germling growth of Sargassum macrocarpum (Phaeophyta) from Ohno-Seto Strait, Hiroshima Bay. Bull Nansei Natl Fish Res Inst. 30:137–145.
Yoshida, T., Sawada, T. & Higaki, M. 1963.
Sargassum vegetation growing in the sea around Tsuyazaki, North Kyushu, Japan. Pac Sci. 17:135–144.
Zandi, K., Ahmadzadeh, S., Tajbakhsh, S., Rastian, Z., Yousefi, F., Farshadpour, F. & Sartavi, K. 2010. Anticancer activity of Sargassum oligocystum water extract against human cancer cell lines. Eur Rev Med Pharmacol Sci. 14:669–673.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||