ABSTRACTRecently, the heterotrophic nanoflagellate Katablepharis japonica has been reported to feed on diverse red-tide species and contribute to the decline of red tides. However, if there are effective predators feeding on K. japonica, its effect on red tide dynamics may be reduced. To investigate potential effective protist predators of K. japonica, feeding by the engulfment-feeding heterotrophic dinoflagellates (HTDs) Oxyrrhis marina, Gyrodinium dominans, Gyrodinium moestrupii, Polykrikos kofoidii, and Noctiluca scintillans, the peduncle-feeding HTDs Luciella masanensis and Pfiesteria piscicida, the pallium-feeding HTD Oblea rotunda, and the naked ciliates Strombidium sp. (approximately 20 μm in cell length), Pelagostrobilidium sp., and Miamiensis sp. on K. japonica was explored. We found that none of these heterotrophic protists fed on actively swimming cells of K. japonica. However, O. marina, G. dominans, L. masanensis, and P. piscicida were able to feed on heat-killed K. japonica. Thus, actively swimming behavior of K. japonica may affect feeding by these heterotrophic protists on K. japonica. To the contrary, K. japonica was able to feed on O. marina, P. kofoidii, O. rotunda, Miamiensis sp., Pelagostrobilidium sp., and Strombidium sp. However, the specific growth rates of O. marina did not differ significantly among nine different K. japonica concentrations. Thus, K. japonica may not affect growth of O. marina. Our findings suggest that the effect of predation by heterotrophic protists on K. japonica might be negligible, and thus, the effect of grazing by K. japonica on populations of red-tide species may not be reduced by mortality due to predation by protists.
INTRODUCTIONMarine heterotrophic nanoflagellates (HNFs) are major components of marine planktonic food webs (e.g., Patterson and Larsen 1991). They are known to be major predators of marine bacteria (Fenchel 1982, Azam et al. 1983, Sieburth 1984). Recently, the free-living HNF Katablepharis japonica has been reported to feed on red-tide dinoflagellates and raphidophytes (Kwon et al. 2017). In turn, some HNFs such as Cafeteria spp., Spumella spp., and Bodo spp. are known to be prey for some heterotrophic dinoflagellates (HTDs) and ciliates (Jürgens et al. 1996, Jeong et al. 2007c). Therefore, they are known to play an important role in the transfer of bacteria and microalgae to HTDs and ciliates in marine microbial loops. However, still feeding by common heterotrophic protists on several important HNF species that can affect the dynamics of red-tide species or bacteria is poorly understood until date.
K. japonica is a common HNF in Korean waters and was found in the waters of Japan and the United States (Okamoto and Inouye 2005, Kahn et al. 2014, Kwon et al. 2017). Recently, this species has been reported to feed on diverse phytoplankton species (Kwon et al. 2017). Interestingly, it can grow fast by feeding on suitable phytoplankton species, but it does not grow on bacteria, unlike most HNFs (Kwon et al. 2017). Furthermore, it was suggested that K. japonica may have a considerable potential grazing impact on populations of a red-tide species. However, if its mortality due to predation is high, the effect of K. japonica on populations of the red-tide species could be reduced. To the best of our knowledge, no studies have been reported on the protist predators of K. japonica. To understand the roles of K. japonica in red tide dynamics, mortality due to predation should be determined.
In addition, HTDs and ciliates are major components in marine ecosystems (Stoecker et al. 1984, Hansen 1991, Jeong 1999, Levinsen and Nielsen 2002, Sherr and Sherr 2007, Jeong et al. 2010, 2015, Yoo et al. 2013a, Lim et al. 2017b). They feed on diverse types of prey, such as bacteria, phytoplankton including red-tide species, mixotrophic, or heterotrophic protists, eggs and early naupliar stages of metazooplankton, and sometimes control populations of their prey (Hansen 1992, Jeong 1994, Montagnes et al. 1996, Jeong et al. 2004, 2008a, 2008b, Kamiyama and Matsuyama 2005, Turner 2006, Yoo et al. 2013b, Lee et al. 2014a, Jang et al. 2016, Lim et al. 2017b). In general, due to much higher abundances of HTDs or ciliates than those of metazooplankton, effect of grazing on prey populations by the former grazers is usually greater than that by the latter grazers (Kim et al. 2013, Yoo et al. 2013a). Thus, to understand the roles of potential prey species in food webs, feeding by HTDs or ciliates on the prey should be explored.
In general, HTDs and ciliates have two major feeding mechanisms, raptorial feeding and filter feeding (e.g., Fenchel 1980, Hansen and Calado 1999). In raptorial feeding, there are three major feeding mechanisms—engulfment feeding, peduncle feeding, and pallium feeding (Jacobson 1987, Jeong and Latz 1994, Kiφrboe and Titelman 1998, Berge et al. 2008, Jeong et al. 2010, Lim et al. 2014, Lee et al. 2015). Using these diverse feeding mechanisms, they are able to feed on prey species having diverse size, shape, behavior, biochemistry, etc. (Jeong et al. 2010). Therefore, in the present study, we aimed to investigate the potential effects of protist predators on K. japonica, feeding by the engulfment-feeding HTDs Oxyrrhis marina, Gyrodinium dominans, Gyrodinium moestrupii, Polykrikos kofoidii, and Noctiluca scintillans, the peduncle-feeding HTDs Luciella masanensis and Pfiesteria piscicida, the pallium-feeding HTD Oblea rotunda, and the naked ciliates Strombidium sp. (approximately 20 μm in cell length), Pelagostrobilidium sp., and Miamiensis sp. on K. japonica was explored. The strain of K. japonica used in the present study was originally isolated from Masan Bay, where most of these heterotrophic protists were isolated or reported to be abundant at certain times (Jeong et al. 2007a, 2007b, Yoo et al. 2013a, Kwon et al. 2017). Furthermore, these heterotrophic protists have often been found in the waters of many other countries as well (Strom and Buskey 1993, Watts et al. 2010, Moestrup et al. 2014, Grzebyk et al. 2017). Some dinoflagellates are known to feed on, kill, lyse, or immobilize some common heterotrophic protists (Kim et al. 2017, Ok et al. 2017). Therefore, we investigated whether K. japonica attacks common heterotrophic protists or not. The results of the present study provide a basis for understanding the interactions between K. japonica and common heterotrophic protist species, and their ecological roles in the marine planktonic community.
MATERIALS AND METHODSPreparation of experimental organismsA clonal culture of K. japonica KJMS1610, which was isolated from plankton samples collected from Masan Bay, Korea in October 2016, was used in the present study (Kwon et al. 2017). The mixotrophic dinoflagellate Akashiwo sanguinea was provided as prey every day. As the concentration of K. japonica increased, the culture was sequentially transferred to 50-, 250-, and 800-mL flasks containing fresh prey (approximately 10,000 cells mL−1). The flasks were filled to capacity with freshly filtered seawater, capped, and placed on a shelf at 20°C under an illumination of 20 μE m−2 s−1 provided by cool white fluorescent lights, under a 14 : 10 h light : dark cycle. The mean equivalent spherical diameter and carbon content of K. japonica were 8.1 μm and 0.043 ng C per cell, respectively (Kwon et al. 2017).
For the isolation and culture of the HTD predators G. dominans, G. moestrupii, L. masanensis, N. scintillans, O. rotunda, O. marina, and P. kofoidii, plankton samples were collected by using water samplers, from the coastal waters off Saemankeum, Jangheung, Jinhae, or Shiwha, Korea during 2008–2016 (Table 1). A clonal culture of each species was established by using two serial single-cell isolations (Table 1). A clonal culture of P. piscicida was obtained from National Center for Marine Algae and Microbiota, United States.
For the isolation and culture of the ciliate Strombidium sp. (approximate cell length 20 μm), plankton samples were collected by using a 10-μm mesh from Jinhae Bay, Korea in July 2017, when the water temperature and salinity were 23.8°C and 28.3, respectively (Table 1). A clonal culture of Strombidium sp. was established by using two serial single-cell isolations. For the isolation and culture of the ciliate Pelagostrobilidium sp. (approximate cell length 50 μm), plankton samples were collected by using a 10-μm mesh, in coastal waters off Tongyoung, Korea in August 2017 when the water temperature and salinity were 27.2°C and 31.5, respectively (Table 1). Furthermore, the scuticociliate Miamiensis sp. B1 was isolated from an infected larva of Clithon retropictus in the waters off Bieung Island, western Korea in May 2016, when water temperature and salinity were 20.8°C and 31.3, respectively (Kim et al. 2017).
The carbon contents of the HTDs and the ciliate were estimated from the cell volume, according to the procedure of Menden-Deuer and Lessard (2000). The cell volumes of the predators were estimated using the methods of Kim and Jeong (2004) and Yoon et al. (2012) for G. dominans and G. moestrupii, that of Jeong et al. (2008b) for O. marina, Jeong et al. (2001b) for P. kofoidii, Jeong et al. (2007a, 2007b) for L. masanensis and P. piscicida, and the cell volumes of N. scintillans, O. rotunda, Strombidium sp., Pelagostrobilidium sp., and Miamiensis sp. were measured in this study (Table 1).
Interactions between Katablepharis japonica and heterotrophic protistsExperiment (Exp.) 1 was designed to investigate interactions between K. japonica and each of the heterotrophic protists after two components were mixed (Table 2). In this experiment, it was tested whether the target heterotrophic protist is able to feed on K. japonica and vice versa.
A culture of approximately 8,000 cells mL−1 of K. japonica growing on A. sanguinea was transferred to a single 250-mL culture flask containing freshly filtered seawater after A. sanguinea became undetectable. This culture was maintained for 1 day. Three 1-mL aliquots were then removed from the flask and examined with a light microscope to determine the concentration of K. japonica.
The initial concentrations of K. japonica (approximately 5,000–8,000 cells mL−1) and each of the target heterotrophic protist species were established using an autopipette to deliver a predetermined volume of culture with a known cell density into the wells of 6-well plate chambers. For each heterotrophic protist species, duplicate wells containing mixtures of K. japonica and heterotrophic protist (a total of 5 mL in each well), duplicate predator control wells containing a culture of heterotrophic protists only (a total of 5 mL in each well), and duplicate prey control wells containing a culture of K. japonica only (a total of 5 mL in each well) were established. The plate chambers were placed on a shelf and incubated under a 14 : 10 h light : dark cycle of 20 μE m−2 s−1 of cool white fluorescent light.
After 2 and 24 h, >30 target predator cells in the well was monitored using an inverted epifluorescence microscope equipped with differential interference contrast at a magnification of 50–200× to determine whether the target predator species is able to feed on K. japonica or not. The feeding process of each of these heterotrophic protists on K. japonica was recorded using a video analyzing system (Sony DXC-C33; Sony Co., Tokyo, Japan) and also captured using a digital camera. The reciprocal feeding of K. japonica on each of the heterotrophic protists was tested and recorded in the same manners as described above.
Exp. 2 was designed to investigate the interactions between heat-killed K. japonica cells and each of the heterotrophic protists after the two taxa were mixed (Table 2). To prepare heat-killed K. japonica cells, a dense culture of K. japonica (approximately 5,000–8,000 cells mL−1) was added to a conical tube, which was then placed in a 100-mL beaker containing 60°C water for 40 s. The heat-killed K. japonica cells were confirmed to be motionless, but not decomposed, under a dissecting microscope. After 2 and 24 h of incubation, >30 cells of each predator in the plate chambers were observed as in Exp. 1, under an epifluorescence microscope at a magnification of 50–200×, to determine whether the predator was able to feed on the heat-killed K. japonica cells.
Effects of the Katablepharis japonica concentration on the growth rate of Oxyrrhis marinaIn Exp. 1, there was no heterotrophic protist that is capable of feeding on actively swimming K. japonica. To the contrary, K. japonica fed on O. marina. Thus, Exp. 3 was designed to measure the growth rate of O. marina as a function of K. japonica concentration (Table 2).
For the experiment on A. sanguinea prey, cultures of approximately 11,000 cells mL−1 of K. japonica growing on A. sanguinea were transferred to a single 250-mL culture flask containing freshly filtered seawater after A. sanguinea became undetectable. This culture was maintained for 1 day. Three 1-mL aliquots were then collected from the flask and examined using a light microscope to determine the concentration of K. japonica.
The initial concentrations of K. japonica and A. sanguinea were established using an autopipette to deliver predetermined volumes of known cell concentrations to the flasks. For each predator-prey combination, triplicate experimental 50-mL culture flasks (containing a mixture of K. japonica and O. marina) and triplicate control flasks (containing a culture of K. japonica only) were established. Moreover, triplicate control flasks containing only O. marina were established at a single concentration. The flasks were filled to 20 mL with freshly filtered seawater, capped, and then placed on a shelf at 20°C under a 14 : 10 h light : dark cycle of 20 μE m−2 s−1 of cool white fluorescent light. To determine the actual initial predator and prey densities (cells mL−1) at the beginning of the experiment (Table 2) and after a 2-day incubation period, 10-mL aliquots were removed from each flask and fixed with 5% Lugol’s solution. Then, all K. japonica cells and all or >300 prey cells in three 1-mL Sedgwick-Rafter chambers were enumerated. Only 10 mL water in each 50-mL flask after subsampling at the beginning of the experiment was incubated to create a shallow depth in the flasks for increasing the encounter rates between O. marina and K. japonica, because K. japonica swam near the bottom of the flask.
The specific growth rate of O. marina was calculated as follows:
where C0 is the initial concentration of O. marina and Ct is the final concentration after time t. The period was 2 days. The mean prey concentration was calculated using the equation of Frost (1972). The ingestion and clearance rates were calculated using the equations of Frost (1972) and Heinbokel (1978).
RESULTSInteractions between Katablepharis japonica and common heterotrophic protistsFor 24 h, none of the heterotrophic protists tested in this study fed on actively swimming cells of K. japonica successfully (Table 3). In video recording for ca. 1 h, there were many encounters between K. japonica and each of the heterotrophic protists studied, but feeding by the heterotrophic protists on K. japonica were not observed (Figs 1 & 2, Supplementary videos S1 & S2). Interestingly, O. marina, G. dominans, G. moestrupii, L. masanensis, P. piscicida, Pelagostrobilidium sp., Strombidium sp., and Miamiensis sp. attempted to feed on them, but N. scintillans, O. rotunda, and P. kofoidii did not attempt to feed on K. japonica cells (Table 3).
When heat-killed K. japonica cells were provided, O. marina, G. dominans, L. masanensis, and P. piscicida were able to feed on them, but the other heterotrophic protists studied did not feed on the heat-killed cells (Table 3, Fig. 3, Supplementary videos S3 & S4).
To the contrary, K. japonica were able to feed on actively swimming cells of O. marina, P. kofoidii, O. rotunda, Miamiensis sp., Pelagostrobilidium sp., and Strombidium sp., but it did not feed on G. dominans, G. moestrupii, L. masanensis, N. scintillans, and P. piscicida (Table 3, Figs 4 & 5, Supplementary videos S5–S10). Only a few K. japonica cells first attacked a HTD prey cell or a ciliate cell, but many K. japonica cells approached and attacked together (Figs 4 & 5). After a few K. japonica cells attacked a heterotrophic protist cell, the attacked cells almost disappeared within ca. 60 s for O. marina, 360 s for P. kofoidii, 280–285 s for Miamiensis sp. and Strombidium sp., 159 s for Pelagostrobilidium sp. and 1,200 s for O. rotunda (Figs 4 & 5).
Effects of the Katablepharis japonica concentration on the growth rate of Oxyrrhis marinaWhen nine different K. japonica concentrations were provided, the specific growth rates of O. marina were not significantly different from one another (p > 0.1, ANOVA) (Fig. 6).
DISCUSSIONInteractions between Katablepharis japonica and common heterotrophic protistsThe present study clearly showed that no common HTDs and ciliates fed on actively swimming K. japonica cells. Recently, K. japonica has been reported to be an effective grazer on red-tide organisms (Kwon et al. 2017). There are many dinoflagellate grazers that feed on red-tide organisms (Stoecker 1999, Tillmann 2004, Jeong et al. 2005, 2010, 2012, 2015, Park et al. 2006, Burkholder et al. 2008, Lee et al. 2014b, 2016, Johnson 2015, Kim et al. 2015, Lim et al. 2015, 2017a, Jang et al. 2017). However, unlike K. japonica, some of these dinoflagellate grazers are in turn fed on by other dinoflagellates and ciliates (Hansen 1992, Jeong and Latz 1994, Nakamura et al. 1995, Jacobson and Anderson 1996, Buskey 1997, Jeong et al. 1999, 2004, 2014, Naustvoll 2000, Kim and Jeong 2004, Adolf et al. 2007, Berge et al. 2008, Jang et al. 2016). Thus, K. japonica may have an advantage and outcompete some dinoflagellate grazers. Furthermore, negligible mortality rate of K. japonica due to predation by common heterotrophic protists may enable it to contribute to grazing on red-tide organisms.
Of the HTDs included in the present study, O. marina, G. dominans, L. masanensis, and P. piscicida fed on heat-killed K. japonica cells, whereas G. moestrupii, N. scintillans, O. rotunda, P. kofoidii did not feed on even heat-killed cells. Furthermore, O. marina, G. dominans, L. masanensis, and P. piscicida attempted to feed on them. Therefore, the active swimming behavior of K. japonica is likely to be the primary cause for the lack of feeding by these four HTDs.
The HTDs N. scintillans, O. rotunda, and P. kofoidii did not feed on heat-killed K. japonica cells or attempt to feed on actively swimming K. japonica cells. Before ingesting prey cells, N. scintillans uses a tentacle to capture prey cells. O. rotunda uses a tow filament, and P. kofoidii uses a nematocyst-taeniocyst complex (Strom and Buskey 1993, Kiφrboe and Titelman 1998, Matsuoka et al. 2000, Jeong et al. 2001b). Therefore, K. japonica cells may not draw a use of the tentacle or deployment of a tow filament or a nematocyst-taeniocyst complex.
Interestingly, the ciliates Pelagostrobilidium sp., Strombidium sp., and Miamiensis sp. attempted to feed on actively swimming K. japonica cells, but they did not feed on heat-killed cells. Therefore, these ciliates may need a stimulus from prey cells to attack them. Feeding by some ciliates is known to be affected by mechanical or chemical stimuli of prey cells (e.g., Buskey and Stoecker 1989, Jakobsen et al. 2006).
Cells of K. japonica are able to feed on actively swimming cells of O. marina, P. kofoidii, O. rotunda, Miamiensis sp., Pelagostrobilidium sp., and Strombidium sp. The duration from the time when a few K. japonica cells attacked a heterotrophic protist cell to that when the attacked cells almost disappeared was the shortest for the naked dinoflagellate O. marina (60 s), intermediate for the naked dinoflagellate P. kofoidii and naked ciliates Miamiensis sp., Pelagostrobilidium sp., and Strombidium sp. (159–360 s), but the longest for the thecate dinoflagellate O. rotunda (1,200 s). The cell volumes of P. kofoidii and the ciliates are much greater than that of O. rotunda. Therefore, the presence of the theca of O. rotunda may be partially responsible for the greater duration, as compared to that of the protists that do not have theca.
Effects of the Katablepharis japonica concentration on the growth rate of Oxyrrhis marina
O. marina does not feed on actively swimming K. japonica, but it is able to feed on heat-killed K. japonica cells. Thus, O. marina may feed on dead K. japonica cells in natural environments. However, K. japonica is able to feed on O. marina. Thus, determining growth rate (or survival rate) of O. marina as a function of K. japonica is needed. When suitable prey species are provided, the specific growth rates of O. marina usually increase rapidly and then become saturated with increasing prey concentration. The phototrophic dinoflagellate prey Amphidinium carterae, Azadinium cf. poporum, Biecheleria cincta, Gymnodinium aureolum, Symbiodinium voratum, the HTD Pfiesteria piscicida, the haptophyte Isochrysis galbana, the euglenophyte Eutreptiella gymnastica, and the raphidophyte Heterosigma akashiwo cause this positive trend (Jeong et al. 2001a, 2003, 2007b, 2011, 2014, Kimmance et al. 2006, Yoo et al. 2010, 2013c, Potvin et al. 2013). However, when inhibitory species are provided, with increasing prey concentration, the specific growth rates of O. marina usually decrease; the phototrophic dinoflagellates Alexandrium pohangense, Alexandrium tamarense, Paragymnodinium shiwhaense, and Takayama helix cause this negative trend (Tillmann and John 2002, Kim et al. 2016, Jeong et al. 2017, Ok et al. 2017). However, in the present study, the specific growth rates of O. marina were not significantly different among the nine different K. japonica concentrations. Thus, K. japonica may neither markedly support nor inhibit the growth of O. marina.
Ecological importanceThe results of the present study and those of our previous study (Kwon et al. 2017) suggest that the HNF K. japonica could be an effective grazer on diverse red-tide organisms with negligible mortality due to heterotrophic protist predators. Thus, when establishing models for predicting the process of red tide formation, grazing impact by K. japonica on the red-tide organisms and low mortality of K. japonica should be considered. Furthermore, in the microbial loop, flagellates are positioned between bacteria or algal prey and ciliates (Azam et al. 1983). However, K. japonica is not fed on by ciliates. Thus, K. japonica may not be a link between bacteria or algal prey and ciliates. Moreover, the maximum growth rate of K. japonica is lower than that of the other HNFs (Kwon et al. 2017). Thus, the low mortality rate due to predation by common heterotrophic protists may compensate the relatively low growth rate of K. japonica.
SUPPLEMENTARY MATERIALSSupplementary video S1No feeding by Oxyrrhis marina (Om) on Katablepharis japonica (Kj) (http://www.e-algae.org). Supplementary video S2No feeding by Oblea rotunda (Or) on Katablepharis japonica (Kj) (http://www.e-algae.org). Supplementary video S3Feeding by Oxyrrhis marina (Om) on heat-killed Katablepharis japonica (Kj) (http://www.e-algae.org). Supplementary video S4Feeding by Gyrodinium dominans (Gd) on heat-killed Katablepharis japonica (Kj) (http://www.e-algae.org). Supplementary video S5
Katablepharis japonica (Kj) feeding on Oxyrrhis marina (Om) (http://www.e-algae.org). Supplementary video S6
Katablepharis japonica (Kj) feeding on Polykrikos kofoidii (Pk) (http://www.e-algae.org). Supplementary video S7
Katablepharis japonica (Kj) feeding on Oblea rotunda (Or) (http://www.e-algae.org). Supplementary video S8
Katablepharis japonica (Kj) feeding on Miamiensis sp. (Mia) (http://www.e-algae.org). Supplementary video S9
Katablepharis japonica (Kj) feeding on Strombidium sp. (Stm) (http://www.e-algae.org). Supplementary video S10
Katablepharis japonica (Kj) feeding on Pelagostrobilidium sp. (Pst) (http://www.e-algae.org). ACKNOWLEDGEMENTSWe thank An Suk Lim, Kyung Ha Lee, Jin Hee Ok, and Hee Chang Kang for technical support. This research was supported by the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2015M1A5A1041806) and the Useful Dinoflagellate Program of Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) and Management of marine organisms causing ecological disturbance and harmful effect Program of KIMST, and Research Institute of Oceanography, SNU award to HJJ.
Fig. 1No feeding by engulfment feeding heterotrophic dinoflagellates on Katablepharis japonica recorded using video microscopy for approximately 1 h. (A–C) No feeding by Oxyrrhis marina (Om) on K. japonica (Kj) cells. (D–F) No feeding by Gyrodinium dominans (Gd) on Kj cells. (G–I) No feeding by Gyrodinium moestrupii (Gm) on Kj cells. (J–L) No feeding by Polykrikos kofoidii (Pk) on Kj cells. (M–O) No feeding by Oblea rotunda (Or) on Kj cells. Red arrows and blue arrowheads indicate Kj cells and heterotrophic protists. The numbers indicate h : min : s. Scale bars represent: A–O, 25 μm. 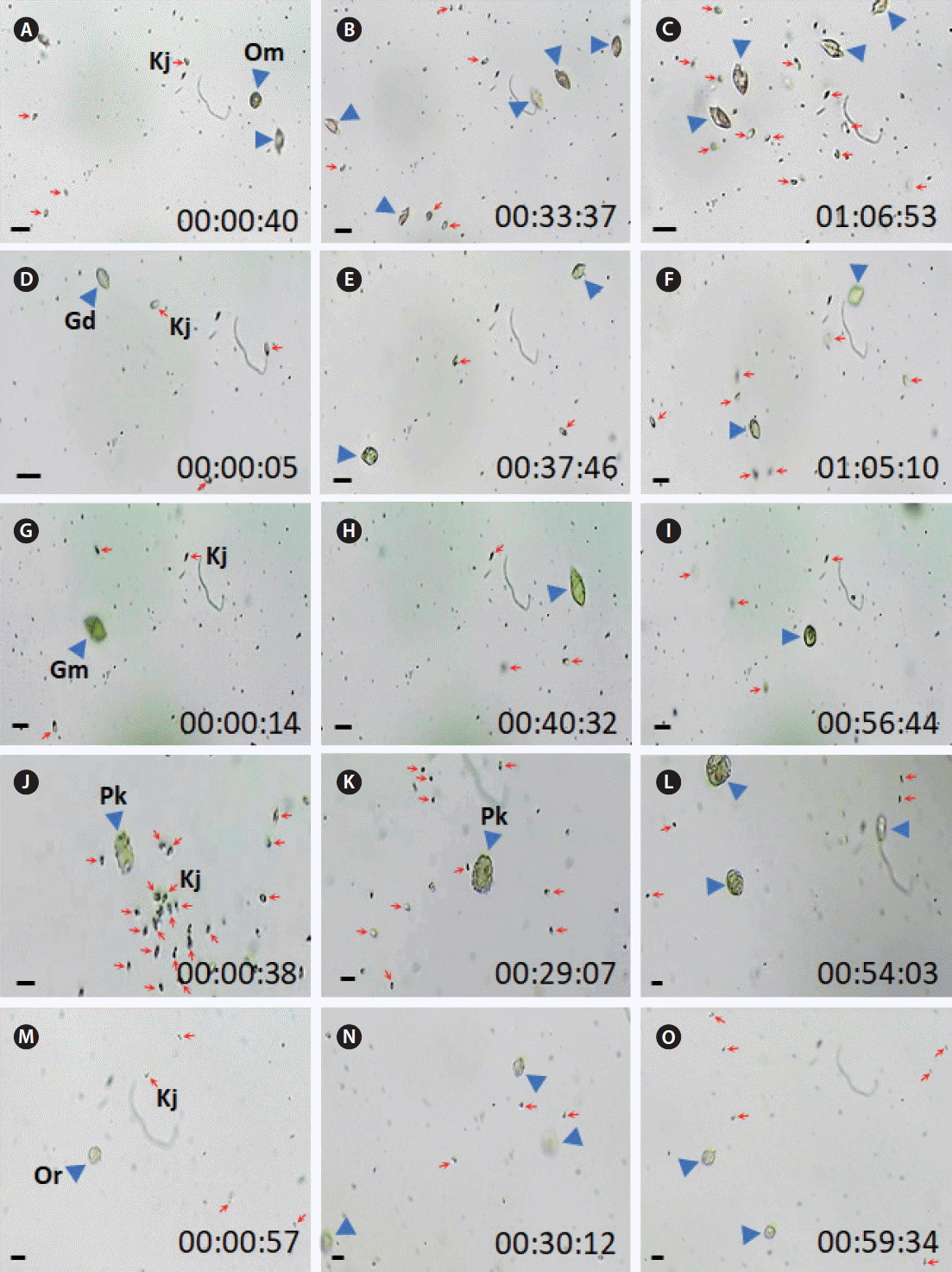
Fig. 2No feeding by tentacle (A–C) and peduncle feeding heterotrophic dinoflagellates (D–I) and ciliates (J–O) on Katablepharis japonica recorded using video microscopy for approximately 1 h. (A–C) No feeding by Noctiluca scintillans (Ns) on K. japonica (Kj) cells. (D–F) No feeding by Pfiesteria piscicida (Pp) on Kj cells. (G–I) No feeding by Luciella masanensis (Lm) on Kj cells. (J–L) No feeding by Miamiensis sp. (Mia) on Kj cells. (M–O) No feeding by Pelagostrobilidium sp. (Pst) on Kj cells. Red arrows and blue arrowheads indicate Kj cells and heterotrophic protists. The numbers indicate h : min : s. Scale bars represent: A–C, 50 μm; D–O, 25 μm. 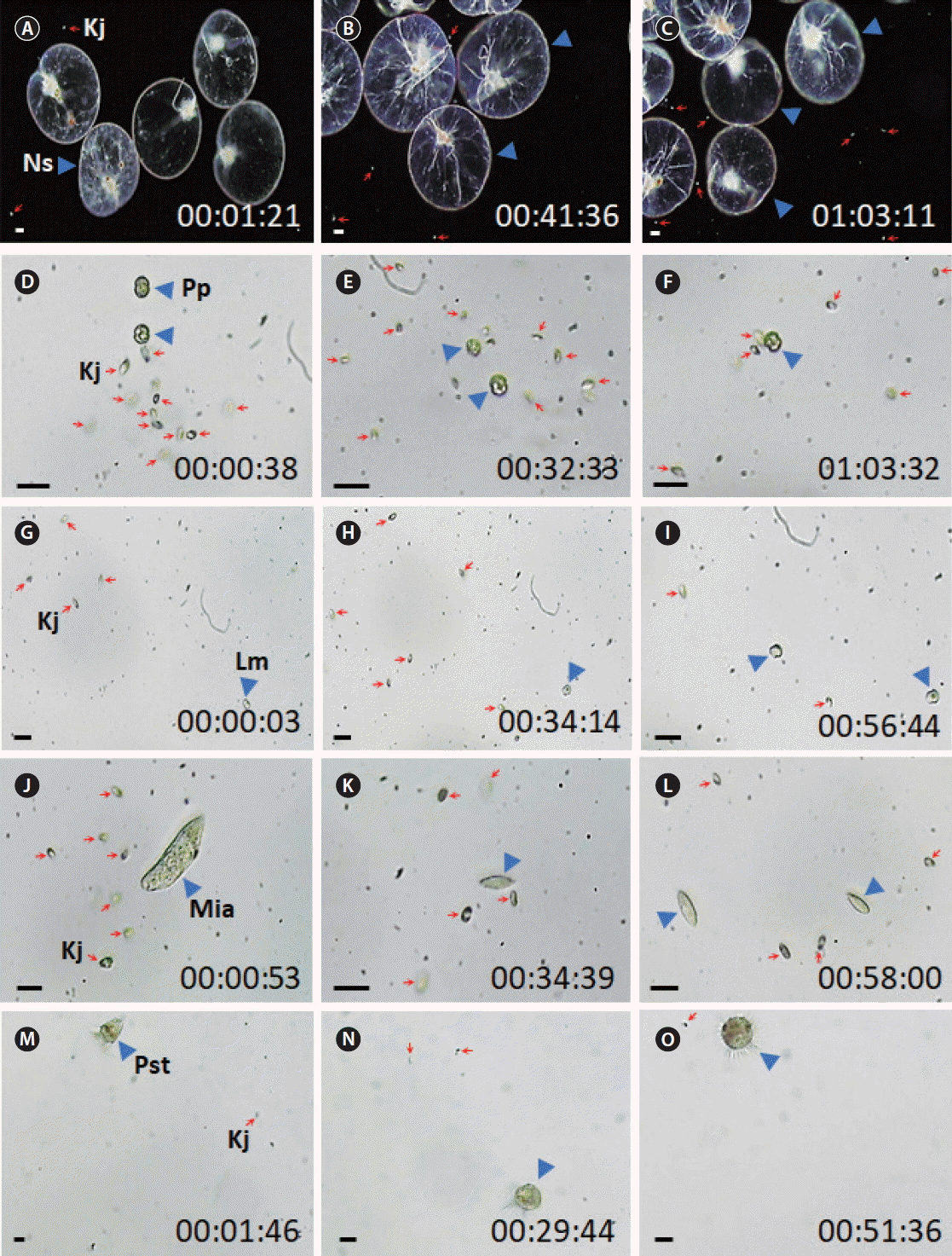
Fig. 3The feeding process of heterotrophic dinoflagellates on heat-killed Katablepharis japonica (Kj) cells recorded using video microscopy. (A–F) Feeding by Oxyrrhis marina (Om) on a heat-killed Kj cell. (G–L) Feeding by Gyrodinium dominans (Gd) on a heat-killed Kj cell. (M-R) Feeding by Pfiesteria piscicida (Pp) on a heat-killed Kj cell. (S–X) Feeding by Luciella masanensis (Lm) on a heat-killed Kj cell. Red arrows and blue arrowheads indicate Kj cells and heterotrophic protists. The numbers indicate min : s. Scale bars represent: A–X, 25 μm. 
Fig. 4The feeding process of Katablepharis japonica (Kj) on heterotrophic dinoflagellates recorded using video microscopy. (A–F) Kj cells feeding an Oxyrrhis marina (Om) cell. (G–L) Kj cells feeding on a Polykrikos kofoidii (Pk) cell. (M–R) Kj cells feeding an Oblea rotunda (Or) cell. Red arrows and blue arrowheads indicate Kj cells and heterotrophic protists. The numbers indicate min : s. Scale bars represent: A–R, 25 μm. 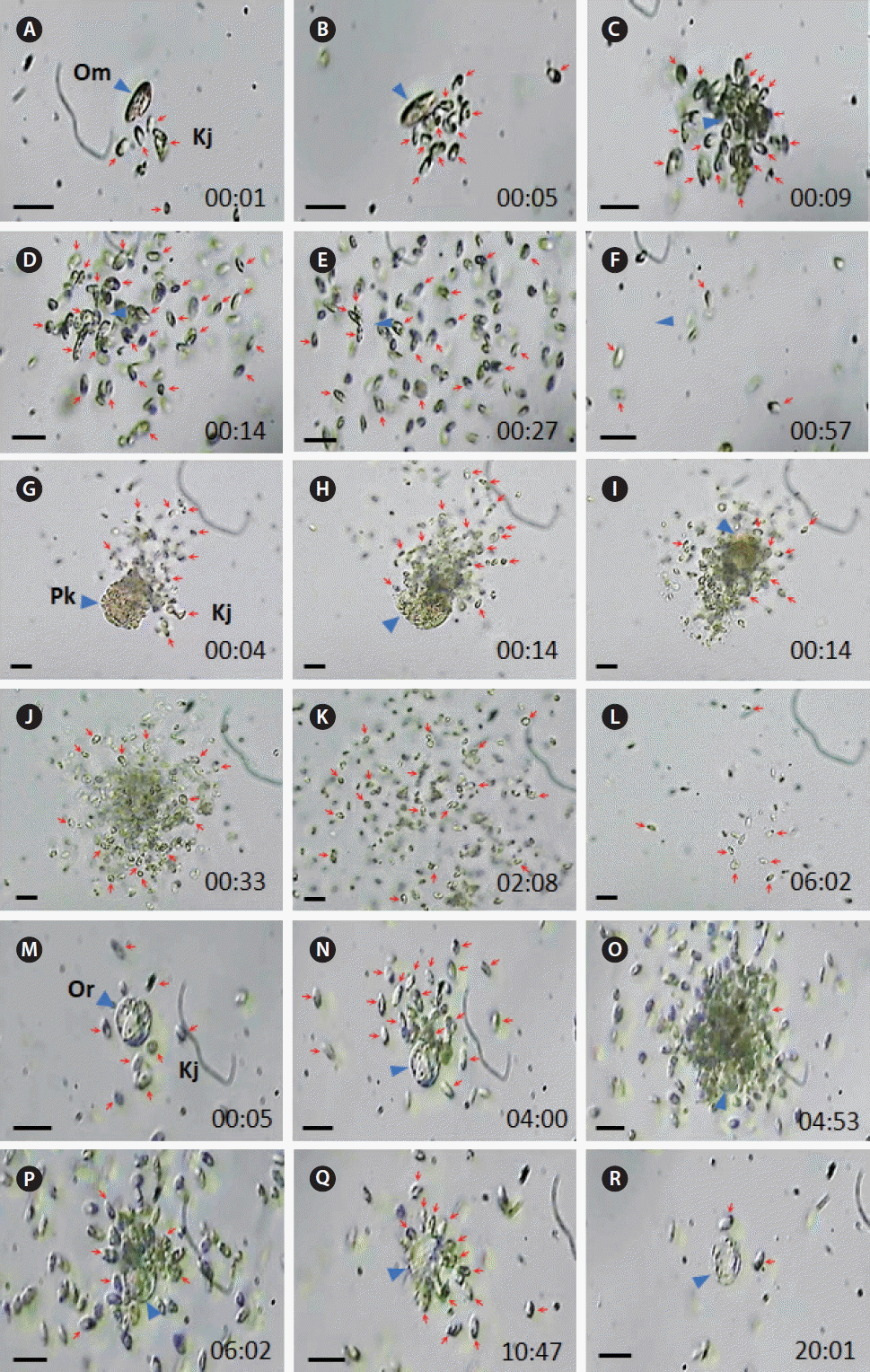
Fig. 5The feeding process of Katablepharis japonica (Kj) on ciliates recorded using video microscopy. (A–F) Kj cells feeding a Miamiensis sp. (Mia) cell. (G–L) Kj cells feeding on a Strombidium sp. (Stm) cell. (M–R) Kj cells feeding an Pelagostrobilidium sp. (Pst) cell. Red arrows and blue arrowheads indicate Kj cells and heterotrophic protists. The numbers indicate min : s. Scale bars represent: A–R, 25 μm. 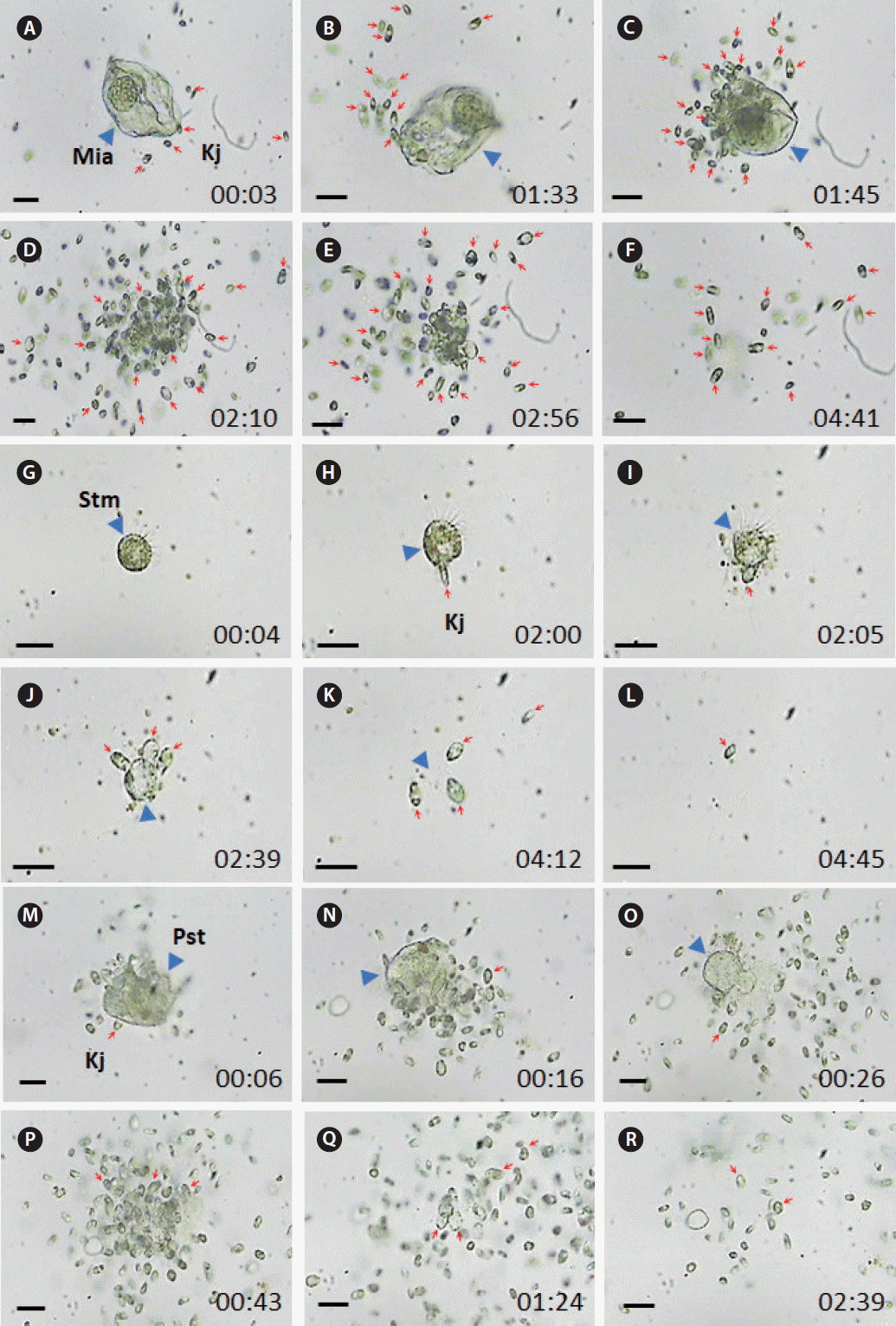
Fig. 6Specific growth rates of Oxyrrhis marina as a function of mean Katablepharis japonica (Kj) concentration. Symbols represent treatment means ± 1 standard error. No difference in the growth rates was observed at the different Kj concentrations (p > 0.1, ANOVA). 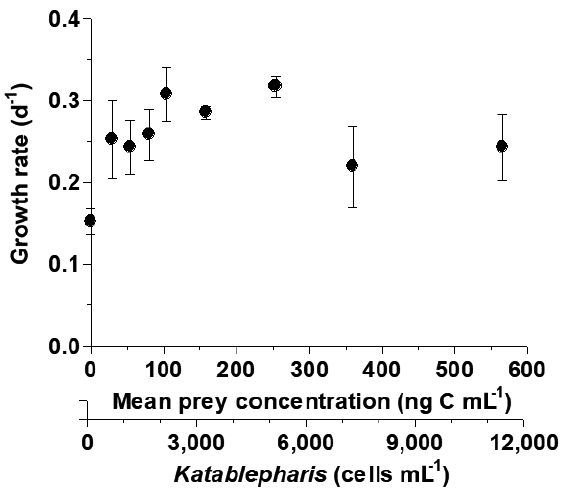
Table 1Isolation and maintenance conditions for the experimental organisms Table 2Experimental design Table 3Taxa, size, and concentration of potential heterotrophic dinoflagellate and naked ciliate predators offered to Katablepharis japonica
REFERENCESAdolf, JE., Krupatkina, D., Bachvaroff, T. & Place, AR. 2007. Karlotoxin mediates grazing by Oxyrrhis marina on strains of Karlodinium veneficum
. Harmful Algae. 6:400–412.
Azam, F., Fenchel, T., Field, JG., Gray, JS., Meyer-Reil, LA. & Thingstad, F. 1983. The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser. 10:257–263.
Berge, T., Hansen, PJ. & Moestrup, Ø. 2008. Feeding mechanism, prey specificity and growth in light and dark of the plastidic dinoflagellate Karlodinium armiger
. Aquat Microb Ecol. 50:279–288.
Burkholder, JM., Glibert, PM. & Skelton, HM. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae. 8:77–93.
Buskey, EJ. 1997. Behavioral components of feeding selectivity of the heterotrophic dinoflagellate Protoperidinium pellucidum
. Mar Ecol Prog Ser. 153:77–89.
Buskey, EJ. & Stoecker, DK. 1989. Behavioral responses of the marine tintinnid Favella sp. to phytoplankton: influence of chemical, mechanical and photic stimuli. J Exp Mar Biol Ecol. 132:1–16.
Fenchel, T. 1980. Relation between particle size selection and clearance in suspension-feeding ciliates. Limnol Oceanogr. 25:733–738.
Fenchel, T. 1982. Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar Ecol Prog Ser. 9:35–42.
Frost, BW. 1972. Effects of size and concentration of food particles on the feeding behavior of the marine planktonic copepod Calanus pacificus
. Limnol Oceanogr. 17:805–815.
Grzebyk, D., Audic, S., Lasserre, B., Abadie, E., de Vargas, C. & Bec, B. 2017. Insights into the harmful algal flora in northwestern Mediterranean coastal lagoons revealed by pyrosequencing metabarcodes of the 28S rRNA gene. Harmful Algae. 68:1–16.
Hansen, PJ. 1991. Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagial food web. Mar Ecol Prog Ser. 73:253–261.
Hansen, PJ. 1992. Prey size selection, feeding rates and growth dynamics of heterotrophic dinoflagellates with special emphasis on Gyrodinium spirale
. Mar Biol. 114:327–334.
Hansen, PJ. & Calado, AJ. 1999. Phagotrophic mechanisms and prey selection in free-living dinoflagellates. J Eukaryot Microbiol. 46:382–389.
Heinbokel, JF. 1978. Studies on the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar Biol. 47:177–189.
Jacobson, DM. 1987. The ecology and feeding biology of thecate heterotrophic dinoflagellates. PhD dissertation. Woods Hole Oceanographic Institution/Massachusetts Institute of Technology Joint Program, Flamouth, MA, 210 pp.
Jacobson, DM. & Anderson, DM. 1996. Widespread phagocytosis of ciliates and other protists by marine mixotrophic and heterotrophic thecate dinoflagellates. J Phycol. 32:279–285.
Jakobsen, HH., Everett, LM. & Strom, SL. 2006. Hydromechanical signaling between the ciliate Mesodinium pulex and motile protist prey. Aquat Microb Ecol. 44:197–206.
Jang, SH., Jeong, HJ., Kwon, JE. & Lee, KH. 2017. Mixotrophy in the newly described dinoflagellate Yihiella yeosuensis: a small, fast dinoflagellate predator that grows mixotrophically, but not autotrophically. Harmful Algae. 62:94–103.
Jang, SH., Jeong, HJ., Lim, AS., Kwon, JE. & Kang, NS. 2016. Feeding by the newly described heterotrophic dinoflagellate Aduncodinium glandula: having the most diverse prey species in the family Pfiesteriaceae. Algae. 31:17–31.
Jeong, HJ. 1994. Predation by the heterotrophic dinoflagellate Protoperidinium cf. divergens on copepod eggs and early naupliar stages. Mar Ecol Prog Ser. 114:203–208.
Jeong, HJ. 1999. The ecological roles of heterotrophic dinoflagellates in marine planktonic community. J Eukaryot Microbiol. 46:390–396.
Jeong, HJ., Ha, JH., Yoo, YD., Park, JY., Kim, JH., Kang, NS., Kim, TH., Kim, HS. & Yih, WH. 2007a. Feeding by the Pfiesteria-like heterotrophic dinoflagellate Luciella masanensis
. J Eukaryot Microbiol. 54:231–241.
Jeong, HJ., Kang, H., Shim, JH., Park, JK., Kim, JS., Song, JY. & Choi, H-J. 2001a. Interactions among the toxic dinoflagellate Amphidinium carterae, the heterotrophic dinoflagellate Oxyrrhis marina, and the calanoid copepods Acartia spp. Mar Ecol Prog Ser. 218:77–86.
Jeong, HJ., Kim, JS., Lee, KH., Seong, KA., Yoo, YD., Kang, NS., Kim, TH., Song, JY. & Kwon, JE. 2017. Differential interactions between the nematocyst-bearing mixotrophic dinoflagellate Paragymnodinium shiwhaense and common heterotrophic protists and copepods: killer or prey. Harmful Algae. 62:37–51.
Jeong, HJ., Kim, JS., Song, JY., Kim, JH., Kim, TH., Kim, SK. & Kang, NS. 2007b. Feeding by protists and copepods on the heterotrophic dinoflagellates Pfiesteria pisicicida, Stoeckeria algicida, and Luciella masanensis
. Mar Ecol Prog Ser. 349:199–211.
Jeong, HJ., Kim, JS., Yoo, YD., Kim, ST., Kim, TH., Park, MG., Lee, CH., Seong, KA., Rang, NS. & Shim, JH. 2003. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: a potential biological method to control red tides using mass-cultured grazers. J Eukaryot Microbiol. 50:274–282.
Jeong, HJ., Kim, JS., Yoo, YD., Kim, ST., Song, JY., Kim, TH., Seong, KA., Kang, NS., Kim, MS., Kim, JH., Kim, S., Ryu, J., Lee, HM. & Yih, WH. 2008a. Control of the harmful alga Cochlodinium polykrikoides by the naked ciliate Strombidinopsis jeokjo in mesocosm enclosures. Harmful Algae. 7:368–377.
Jeong, HJ., Kim, SK., Kim, JS., Kim, ST., Yoo, YD. & Yoon, JY. 2001b. Growth and grazing rates of the heterotrophic dinoflagellate Polykrikos kofoidii on red-tide and toxic dinoflagellates. J Eukaryot Microbiol. 48:298–308.
Jeong, HJ., Kim, TH., Yoo, YD., Yoon, EY., Kim, JS., Seong, KA., Kim, KY. & Park, JY. 2011. Grazing impact of heterotrophic dinoflagellates and ciliates on common red-tide euglenophyte Eutreptiella gymnastica in Masan Bay, Korea. Harmful Algae. 10:576–588.
Jeong, HJ. & Latz, MI. 1994. Growth and grazing rates of the heterotrophic dinoflagellates Protoperidinium spp. on red tide dinoflagellates. Mar Ecol Prog Ser. 106:173–185.
Jeong, HJ., Lim, AS., Franks, PJS., Lee, KH., Kim, JH., Kang, NS., Lee, MJ., Jang, SH., Lee, SY., Yoon, EY., Park, JY., Yoo, YD., Seong, KA., Kwon, JE. & Jang, TY. 2015. A hierarchy of conceptual models of red-tide generation: nutrition, behavior, and biological interactions. Harmful Algae. 47:97–115.
Jeong, HJ., Lim, AS., Yoo, YD., Lee, MJ., Lee, KH., Jang, TY. & Lee, K. 2014. Feeding by heterotrophic dinoflagellates and ciliates on the free-living dinoflagellate Symbiodinium sp. (Clade E). J Eukaryot Microbiol. 61:27–41.
Jeong, HJ., Seong, KA., Yoo, YD., Kim, TH., Kang, NS., Kim, S., Park, JY., Kim, JS., Kim, KH. & Song, JY. 2008b. Feeding and grazing impact by small marine heterotrophic dinoflagellates on heterotrophic bacteria. J Eukaryot Microbiol. 55:271–288.
Jeong, HJ., Shim, JH., Lee, CW., Kim, JS. & Koh, SM. 1999. Growth and grazing rates of the marine planktonic ciliate Strombidinopsis sp. on red-tide and toxic dinoflagellates. J Eukaryot Microbiol. 46:69–76.
Jeong, HJ., Song, JE., Kang, NS., Kim, S., Yoo, YD. & Park, JY. 2007c. Feeding by heterotrophic dinoflagellates on the common marine heterotrophic nanoflagellate Cafeteria sp. Mar Ecol Prog Ser. 333:151–160.
Jeong, HJ., Yoo, YD., Kang, NS., Lim, AS., Seong, KA., Lee, SY., Lee, MJ., Lee, KH., Kim, HS., Shin, W., Nam, SW., Yih, W. & Lee, K. 2012. Heterotrophic feeding as a newly identified survival strategy of the dinoflagellate Symbiodinium
. Proc Natl Acad Sci U S A. 109:12604–12609.
Jeong, HJ., Yoo, YD., Kim, JS., Kang, NS., Kim, TH. & Kim, JH. 2004. Feeding by the marine planktonic ciliate Strombidinopsis jeokjo on common heterotrophic dinoflagellates. Aquat Microb Ecol. 36:181–187.
Jeong, HJ., Yoo, YD., Kim, JS., Seong, KA., Kang, NS. & Kim, TH. 2010. Growth, feeding, and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J. 45:65–91.
Jeong, HJ., Yoo, YD., Park, JY., Song, JY., Kim, ST., Lee, SH., Kim, KY. & Yih, WH. 2005. Feeding by the phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat Microb Ecol. 40:133–150.
Johnson, MD. 2015. Inducible mixotrophy in the dinoflagellate Prorocentrum minimum
. J Eukaryot Microbiol. 62:431–443.
Jürgens, K., Wickham, SA., Rothhaupt, KO. & Santer, B. 1996. Feeding rates of macro-and microzooplankton on heterotrophic nanoflagellates. Limnol Oceanogr. 41:1833–1839.
Kahn, P., Herfort, L., Peterson, TD. & Zuber, P. 2014. Discovery of a Katablepharis sp. in the Columbia River estuary that is abundant during the spring and bears a unique large ribosomal subunit sequence element. MicrobiologyOpen. 3:764–776.
Kamiyama, T. & Matsuyama, Y. 2005. Temporal changes in the ciliate assemblage and consecutive estimates for their grazing effect during the course of a Heterocapsa circularisquama bloom. J Plankton Res. 27:303–311.
Kim, JH., Jeong, HJ., Lim, AS., Kwon, JE., Lee, KH., Park, KH. & Kim, HS. 2017. Removal of two pathogenic scuticociliates Miamiensis avidus and Miamiensis sp. using cells or culture filtrates of the dinoflagellate Alexandrium andersonii
. Harmful Algae. 63:133–145.
Kim, JH., Jeong, HJ., Lim, AS., Rho, JR. & Lee, SB. 2016. Killing potential protist predators as a survival strategy of the newly described dinoflagellate Alexandrium pohangense
. Harmful Algae. 55:41–55.
Kim, JS. & Jeong, HJ. 2004. Feeding by the heterotrophic dinoflagellates Gyrodinium dominans and G. spirale on the red-tide dinoflagellate Prorocentrum minimum
. Mar Ecol Prog Ser. 280:85–94.
Kim, JS., Jeong, HJ., Yoo, YD., Kang, NS., Kim, SK., Song, JY., Lee, MJ., Kim, ST., Kang, JH., Seong, KA. & Yih, WH. 2013. Red tides in Masan Bay, Korea, in 2004–2005: III. Daily variation in the abundance of mesozooplankton and their grazing impacts on red-tide organisms. Harmful Algae. 30(Supple 1):S102–S113.
Kim, S., Yoon, J. & Park, MG. 2015. Obligate mixotrophy of the pigmented dinoflagellate Polykrikos lebourae (Dinophyceae, Dinoflagellata). Algae. 30:35–47.
Kimmance, SA., Atkinson, D. & Montagnes, DJS. 2006. Do temperature–food interactions matter? Responses of production and its components in the model heterotrophic flagellate Oxyrrhis marina
. Aquat Microb Ecol. 42:63–73.
Kiφrboe, T. & Titelman, J. 1998. Feeding, prey selection and prey encounter mechanisms in the heterotrophic dinoflagellate Noctiluca scintillans
. J Plankton Res. 20:1615–1636.
Kwon, JE., Jeong, HJ., Kim, SJ., Jang, SH., Lee, KH. & Seong, KA. 2017. Newly discovered role of the heterotrophic nanoflagellate Katablepharis japonica, a predator of toxic or harmful dinoflagellates and raphidophytes. Harmful Algae. 68:224–239.
Lee, KH., Jeong, HJ., Kwon, JE., Kang, HC., Kim, JH., Jang, SH., Park, JY., Yoon, EY. & Kim, JS. 2016. Mixotrophic ability of the phototrophic dinoflagellates Alexandrium andersonii, A. affine, and A. fraterculus
. Harmful Algae. 59:67–81.
Lee, KH., Jeong, HJ., Yoon, EY., Jang, SH., Kim, HS. & Yih, W. 2014a. Feeding by common heterotrophic dinoflagellates and a ciliate on the red-tide ciliate Mesodinium rubrum
. Algae. 29:153–163.
Lee, MJ., Jeong, HJ., Lee, KH., Jang, SH., Kim, JH. & Kim, KY. 2015. Mixotrophy in the nematocyst–taeniocyst complex-bearing phototrophic dinoflagellate Polykrikos hartmannii.
. Harmful Algae. 49:124–134.
Lee, SK., Jeong, HJ., Jang, SH., Lee, KH., Kang, NS., Lee, MJ. & Potvin, E. 2014b. Mixotrophy in the newly described dinoflagellate Ansanella granifera: feeding mechanism, prey species, and effect of prey concentration. Algae. 29:137–152.
Levinsen, H. & Nielsen, TG. 2002. The trophic role of marine pelagic ciliates and heterotrophic dinoflagellates in arctic and temperate coastal ecosystems: a cross-latitude comparison. Limnol Oceanogr. 47:427–439.
Lim, AS., Jeong, HJ., Jang, TY., Yoo, YD., Kang, NS., Yoon, EY. & Kim, GH. 2014. Feeding by the newly described heterotrophic dinoflagellate Stoeckeria changwonensis: a comparison with other species in the family Pfiesteriaceae. Harmful Algae. 36:11–21.
Lim, AS., Jeong, HJ., Kim, JH., Jang, SH., Lee, MJ. & Lee, K. 2015. Mixotrophy in the newly described dinoflagellate Alexandrium pohangense: a specialist for feeding on the fast-swimming ichthyotoxic dinoflagellate Cochlodinium polykrikoides
. Harmful Algae. 49:10–18.
Lim, AS., Jeong, HJ., Kim, JH. & Lee, SY. 2017a. Control of ichthyotoxic Cochlodinium polykrikoides using the mixotrophic dinoflagellate Alexandrium pohangense: a potential effective sustainable method. Harmful Algae. 63:109–118.
Lim, AS., Jeong, HJ., Seong, KA., Lee, MJ., Kang, NS., Jang, SH., Lee, KH., Park, JY., Jang, TY. & Yoo, YD. 2017b. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: II. Heterotrophic protists and their grazing impacts on red-tide organisms. Algae. 32:199–222.
Matsuoka, K., Cho, H-J. & Jacobson, DM. 2000. Observations of the feeding behavior and growth rates of the heterotrophic dinoflgellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phycologia. 39:82–86.
Menden-Deuer, S. & Lessard, EJ. 2000. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr. 45:569–579.
Moestrup, Ø., Hansen, G., Daugbjerg, N., Lundholm, N., Overton, J., Vestergård, M., Steenfeldt, SJ., Calado, AJ. & Hansen, PJ. 2014. The dinoflagellates Pfiesteria shumwayae and Luciella masanensis cause fish kills in recirculation fish farms in Denmark. Harmful Algae. 32:33–39.
Montagnes, DJS., Berger, JD. & Taylor, FJR. 1996. Growth rate of the marine planktonic ciliate Strombidinopsis cheshiri Snyder and Ohman as a function of food concentration and interclonal variability. J Exp Mar Biol Ecol. 206:121–132.
Nakamura, Y., Suzuki, S. & Hiromi, J. 1995. Population dynamics of heterotiophic dinoflagellates during a Gymnodinium mikimotoi red tide in the Seto Inland Sea. Mar Ecol Prog Ser. 125:269–277.
Naustvoll, L-J. 2000. Prey size spectra and food preferences in thecate heterotrophic dinoflagellates. Phycologia. 39:187–198.
Ok, JH., Jeong, HJ., Lim, AS. & Lee, KH. 2017. Interactions between the mixotrophic dinoflagellate Takayama helix and common heterotrophic protists. Harmful Algae. 68:178–191.
Okamoto, N. & Inouye, I. 2005. The katablepharids are a distant sister group of the Cryptophyta: a proposal for Katablepharidophyta divisio nova/Kathablepharida phylum novum based on SSU rDNA and beta-tubulin phylogeny. Protist. 156:163–179.
Park, MG., Kim, S., Kim, HS., Myung, G., Kang, YG. & Yih, W. 2006. First successful culture of the marine dinoflagellate Dinophysis acuminata
. Aquat Microb Ecol. 45:101–106.
Patterson, DJ. & Larsen, J. 1991. The biology of free-living heterotrophic flagellates. Oxford University Press, NY, 505 pp.
Potvin, É., Hwang, YJ., Yoo, YD., Kim, JS. & Jeong, HJ. 2013. Feeding by heterotrophic protists and copepods on the photosynthetic dinoflagellate Azadinium cf. poporum from western Korean waters. Aquat Microb Ecol. 68:143–158.
Sherr, EB. & Sherr, BF. 2007. Heterotrophic dinoflagellates: a significant component of microzooplankton biomass and major grazers of diatoms in the sea. Mar Ecol Prog Ser. 352:187–197.
Sieburth, JM. 1984. Protozoan bacterivory in pelagic marine waters. In : Hobbie JE, Williams PJ, editors Heterotrophic Activity in the Sea. Plenum Press, NY, 405–444.
Stoecker, DK., Davis, LH. & Anderson, DM. 1984. Fine scale spatial correlations between planktonic ciliates and dinoflagellates. J Plankton Res. 6:829–842.
Strom, SL. & Buskey, EJ. 1993. Feeding, growth, and behavior of the thecate heterotrophic dinoflagellate Oblea rotunda
. Limnol Oceanogr. 38:965–977.
Tillmann, U. 2004. Interactions between planktonic microalgae and protozoan grazers. J Eukaryot Microbiol. 51:156–168.
Tillmann, U. & John, U. 2002. Toxic effects of Alexandrium spp. on heterotrophic dinoflagellates: an allelochemical defence mechanism independent of PSP-toxin content. Mar Ecol Prog Ser. 230:47–58.
Turner, JT. 2006. Harmful algae interactions with marine planktonic grazers. In : Granéli E, Turner JT, editors Ecology of Harmful Algae. Springer-Verlag, Berlin, 259–270.
Watts, PC., Martin, LE., Kimmance, SA., Montagnes, DJS. & Lowe, CD. 2010. The distribution of Oxyrrhis marina: a global disperser or poorly characterized endemic? J. Plankton Res. 33:579–589.
Yoo, YD., Jeong, HJ., Kang, NS., Kim, JS., Kim, TH. & Yoon, EY. 2010. Ecology of Gymnodinium aureolum. II. Predation by common heterotrophic dinoflagellates and a ciliate. Aquat Microb Ecol. 59:257–272.
Yoo, YD., Jeong, HJ., Kim, JS., Kim, TH., Kim, JH., Seong, KA., Lee, SH., Kang, NS., Park, JW., Park, J., Yoon, EY. & Yih, WH. 2013a. Red tides in Masan Bay, Korea in 2004–2005: II. Daily variations in the abundance of heterotrophic protists and their grazing impact on red-tide organisms. Harmful Algae. 30(Suppl 1):S89–S101.
Yoo, YD., Yoon, EY., Jeong, HJ., Lee, KH., Hwang, YJ., Seong, KA., Kim, JS. & Park, JY. 2013b. The newly described heterotrophic dinoflagellate Gyrodinium moestrupii, an effective protistan grazer of toxic dinoflagellates. J Eukaryot Microbiol. 60:13–24.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||