Effects of future climate conditions on photosynthesis and biochemical component of Ulva pertusa (Chlorophyta)
Article information
Abstract
Ulva pertusa, a common bloom-forming green alga, was used as a model system to examine the effects of elevated carbon dioxide (CO2) and temperature on growth and photosynthetic performance. To do this, U. pertusa was grown under four temperature and CO2 conditions; ambient CO2 (400 μatm) and temperature (16°C) (i.e., present), elevated temperature only (19°C) (ET; i.e., warming), elevated CO2 only (1,000 μatm) (EC; i.e., acidification), and elevated temperature and CO2 (ET and EC; i.e., greenhouse), and its steady state photosynthetic performance evaluated. Maximum gross photosynthetic rates (GPmax) were highest under EC conditions and lowest under ET conditions. Further, ET conditions resulted in decreased rate of dark respiration (Rd), but growth of U. pertusa was higher under ET conditions than under ambient temperature conditions. In order to evaluate external carbonic anhydrase (eCA) activity, photosynthesis was measured at 70 μmol photons m−2 s−1 in the presence or absence of the eCA inhibitor acetazolamide (AZ), which inhibited photosynthetic rates in all treatments, indicating eCA activity. However, while AZ reduced U. pertusa photosynthesis in all treatments, this reduction was lower under ambient CO2 conditions (both present and warming) compared to EC conditions (both acidification and greenhouse). Moreover, Chlorophyll a and glucose contents in U. pertusa tissues declined under ET conditions (both warming and greenhouse) in conjunction with reduced GPmax and Rd. Overall, our results indicate that the interaction of EC and ET would offset each other’s impacts on photosynthesis and biochemical composition as related to carbon balance of U. pertusa.
INTRODUCTION
Atmospheric concentrations of carbon dioxide (CO2) have rapidly increased since the time of the Industrial Revolution, leading to changes in the chemical composition of seawater. The A1FI scenario of the Intergovernmental Panel on Climate Change (IPCC) projects that the the atmospheric CO2 concentration will have increased to 970 μatm nearly three times the present concentration by the end of the current century (IPCC 2007). Concomitantly, the average pH of seawater is expected to drop by approximately 0.46 units from current levels, with both CO2 and HCO3− concentrations increasing and CO32− decreasing. These changes caused by increasing atmospheric CO2 will also be associated with increased ocean temperature and acidity, sea-level rise, and more frequent natural disasters (IPCC 2007, Doney et al. 2009). There is general agreement that these impacts will be some of the most notable environmental changes in the ocean during the coming decades (e.g., Hansen et al. 2005).
There are several potential biological implications of the expected environmental changes associated with ocean acidification. For example, current CO2 concentrations, in the form of dissolved inorganic carbon (DIC) in seawater, are not high enough to saturate photosynthesis of marine primary producers, and therefore, photosynthesis in macroalgae and phytoplankton could be saturated under current CO2 conditions by using carbon concentration mechanisms (CCMs) (Raven 1997), such as those associated with the enzyme carbonic anhydrase (CA), which catalyzes dehydration of HCO3− to CO2. However, the exact mechanisms by which this occurs may differ at phylogenetic levels, from ecotypes to phyla (Johnston et al. 1992, Mercado et al. 1997, Brading et al. 2011). Indeed, most macroalgae involve CCMs and thus use HCO3− for photosynthesis; however, photosynthesis dose not generally appear saturated under present DIC conditions (Koch et al. 2013). From previous research, several species of Ulva have shown variation in acclimations to changes in DIC, such as the use of HCO3−, the extent of carbonic anhydrate activity, and DIC saturation states. Indeed, many researchers predicted that the change in ocean CO2 concentrations could directly affect CCM activities in many species because increased CO2 enhances the dependence of carbon by diffusion. In addition, the down-regulation of CCMs appears through decreased HCO3− usage and increased reliance on CO2 utilization, and mechanism that has been supported by many laboratory and in situ experiments (Gao et al. 2012).
Some metabolic changes including photosynthesis, respiration, and enzyme activity occur as a result of increased temperature (Atkin et al. 2000, Atkin and Tjoelker 2003, Vona et al. 2004), which in turn, alters the physiological responses of the macroalgae to environmental changes (Kübler and Davison 1995, Zou and Gao 2013). Simultaneously, increased atmospheric CO2 will result in ocean acidification and many unpredictable effects (Doney et al. 2009). Considerable research was carried out on the impacts of these factors; however, most has focused primarily on the processes of biological calcification, the general impacts on phytoplankton communities, and only a few have focused on selected macroalgae. Further, few of these studies have considered the combined impacts of these environmental changes on a bloom-forming green alga using ecologically relevant conditions (e.g., Xu and Gao 2012). Here, we consider the impact of these factors on U. pertusa using environmentally relevant levels of CO2 and temperature.
Ulva species, the globally important bloom-forming green algae, grow in a wide range of temperatures, ranging from 10–30°C, with the highest growth rates generally occurring at 15–20°C (Taylor et al. 2001). Likewise, the sporelings of U. intestinalis (Enteromorpha intestinalis) grow in a wide range of temperatures, with the highest growth rates occurring at 15 and 20°C (Kim and Lee 1996). Further, the floating U. linza, collected in the early summer from the Yellow Sea, exhibits exponential growth at 10–15°C, and tissue senescence occurs at temperatures over 20°C, but gross photosynthesis (GP) is higher at 20–25°C (Kim et al. 2011, Kang et al. 2016). In U. rigida higher growth occurs below 17°C, whereas growth declines above 17°C (de Casabianca et al. 2002). U. pertusa from eelgrass beds in Korea exhibit high growth rates at temperatures between 10 and 25°C (Choi 2003). Together, these different growth rates reflect the temperature regimes these algae experience in natural habitats, and reflect the seasonality and distribution of each species (Gessner 1970, Innes 1988, Lüning 1990).
Considerable research has demonstrated that photosynthetic rates of marine plants are enhanced by increased CO2 concentrations. However, photosynthetic activity depends not only on CO2 concentration but also on nutrient levels, light and temperature conditions (Zimmerman et al. 1997, Gordillo et al. 2001, 2003, Fu et al. 2007, Zou et al. 2011). For example, the marine picocyanobacteria Synechococcus and Prochlorococcus were examined under four conditions that combined changes in CO2 concentration and temperature (Fu et al. 2007). Growth and photosynthesis of Prochlorococcus were unaffected by increasing CO2 and temperature, whereas growth and photosynthesis in Synechococcus were stimulated by increasing temperature, but showed synergistic responses with increasing CO2 and temperature. The recruitment of algal turfs (mainly Feldmannia spp.) also has shown synergistic responses when exposed to combined future CO2 and temperature conditions, with biomass increased two-fold relative to when the turfs were exposed to the single effects of either CO2 or temperature alone (Connell and Russell 2010). Further, the effective quantum yield of algal turfs also increases when exposed to future CO2 conditions, but decreases when exposed to elevated temperature alone. Olabarria et al. (2013) observed that both individuals and assemblages of rockpool macroalgae exhibit various responses to increased CO2 and high temperature. Specifically, increased CO2 and temperature resulted in decreases in macroalgae assemblage biomass because of changes in productivity and respiration. However, these experiments have not previously been conducted with a green tide forming eukaryotic alga where the impacts on natural communities may be extreme.
Ulva pertusa (Chlorophyta) is a green tide species with outbreaks occurring in many coastal areas of Korea (Kim et al. 2004). Blooms of U. pertusa, are associated with elevated nutrients, especially nitrogen and phosphorous, and partially regulated by temperature, light and biotic factors (Valiela et al. 1997, Giannotti and McGlathery 2001). Despite the extensive understanding of the factors associated with green tide outbreaks, there are a few studies that allow us to predict the potential impacts of elevated atmospheric CO2 levels and consequent climate change on these blooms. In this study we simultaneously evaluate the impacts of both elevated CO2 and temperature on the growth of U. pertusa. We focus on biochemical composition, respiration, photosynthetic responses and growth rates as adaptations of U. pertusa to realistic future climate conditions.
MATERIALS AND METHODS
Sample collection and incubation
U. pertusa was collected in December 2010 from the intertidal zone at Wando (34°19′30″ N, 126°49′50″ E), on the southern coast of Korea. Ulva species, especially U. pertusa and U. linza are predominant in this area during winter. The average water temperature and salinity were 10°C and S = 32, respectively at the time of sampling. The samples were rinsed in filtered seawater to remove macroscopic epiphytes and were maintained in filtered, aerated seawater supplemented with F/2 nutrients (FRITZ Industries Inc., Greenville, TX, USA). Thalli were initially maintained at conditions of 16°C, S = 32, and 70 μmol photons m−2 s−1, and a 12 : 12 h light : dark cycle for 3 days prior to experimental initiation.
Experimental design
Four treatments were used to measure the individual and combined effects of CO2 and temperature: ambient CO2 (400 μatm) and temperature (16°C) (i.e., present); ambient CO2 and elevated temperature (19°C) (ET; i.e., warming); elevated CO2 (1,000 μatm) and ambient temperature (EC; i.e., acidification); and both elevated temperature and CO2 (ET and EC combined; i.e., greenhouse). Different CO2 concentrations were provided by mixing air with the appropriate amounts of CO2 obtained from a compressed CO2 cylinder, as described by Kim et al. (2008). Carbon dioxide (CO2) concentrations were monitored in the resulting air-CO2 mixtures using a CO2 analyzer (LI-840A; LI-COR, Lincolon, NE, USA). These mixtures were bubbled through the media, and CO2 was monitored daily via measurements of the pH of culture media using a pH-meter (Meterlab PHM210; Radiometer Analytical SAS, Lyon, France). The maximum variation of CO2 in individual cultures was 5%, and CO2 inputs were adjusted daily in order to maintain constant levels in each experimental treatment.
Total seawater alkalinity (TA) and total DIC were measured with a potentiometric titration system (765 Dosimat; Metrohm AG, Herisau, Switzerland), combined with a ROSS half-cell pH electrode (Orion 8101BNWP; Thermo Scientific, Waltham, MA, USA) and a sure-flow reference electrode (Orion 900200; Thermo Scientific). Proportions of the carbon species in the seawater were calculated from the TA and DIC values using CO2SYS software (Lewis and Wallace 1998). The TA and DIC measurements were checked for accuracy against certified reference materials (distributed by A. Dickson, Scripps Institution of Oceanography) used here as standards. The precisions of the measurement were approximately ±2 μmol kg−1 for TA and ±1.5 μmol kg−1 for DIC. Temperature and irradiance were also monitored using a temperature/light data logger (UA-002-64; Onset, Pocasset, MA, USA) calibrated with a thermometer during the experiment. The experimental temperatures were controlled using aquarium heaters (EHEIM Jager, Deizisau, Germany). Temperatures were maintained to within 0.1°C of the targeted temperature throughout the experiment.
Photosynthetic rates
Photosynthetic rates were measured using an oxygen closed-chamber method consisting of a 2-mm oxygen dipping probe (DP-PSt3) connected to Fibox3 (PreSens, Regenburg, Germany) at eight irradiances (0, 10, 45, 80, 150, 245, and 450 μmol photons m−2 s−1) derived from a halogen lamp (KL2500; SCHOTT, Mainz, Germany). Temperature and CO2 concentrations were maintained at each target level during the photosynthetic measurement. The photosynthesis-irradiance relationships for distinction of photosynthetic traits were fitted to a nonlinear mathematical function that represented the double exponential function (Platt et al. 1980).
External CA activities
In order to assess the external carbonic anhydrase (eCA) activity, photosynthesis was measured with and without acetazolamide (AZ) under 70 μmol photons m−2 s−1. AZ inhibits photosynthesis using HCO3−, hence the results show CO2 utilized during photosynthesis when AZ was added to the seawater media. AZ was prepared in 0.5 N NaOH and added to the medium at a concentration of 60 μM. Experiments with AZ used the same irradiance levels as were used in the incubation conditions.
Chlorophyll a (Chl a) fluorescence
Chl a fluorescence was measured using Diving-PAM (Walz GmbH, Effeltrich, Germany). Effective quantum yield (ΦPSII) was calculated as:
where F and Fm′ represent the steady-state fluorescence and maximum fluorescence measured in the light, respectively. Rapid light curves (RLC) were determined according to Ralph and Gademann (2005). Relative electron transport rate (rETR) was calculated as:
with two ambiguous factors (the partitioning of light energy by photosystem [PS] I and PSII, and the absorption factor) were not considered. Chl a fluorescence parameters from rETR-irradiance curves were calculated using a nonlinear regression equation (Platt et al. 1980).
Growth rates
U. pertusa thalli were prepared by slicing individual 20 mm diameter disks from near the base of each thallus using a cork borer. To reduce the effects of damage at the disk margins, disks were cut one day prior to the start of the growth experiment. The growth rate (μ) was calculated as:
where A and A0 represent the areas at time T (after 10 days) and T0 (the initial day), respectively.
Chl a, tissue nutrient, and glucose contents
Chl a contents were determined using a spectrophotometer (Helios α UV-Vis; Unicam, Cambridge, UK). Each U. pertusa disk that was used to evaluate growth above was extracted in 20 mL glass vials with 8 mL of N,N-dimethylformamide at 4°C for 24 h in the dark. The Chl a content was calculated as:
where A664.5 and A647 represent absorbance at 664.5 nm and 647 nm, respectively (Inskeep and Bloom 1985).
Carbon (C) and nitrogen (N) contents within the U. pertusa tissues were determined from individual disks using an elemental analyzer (EA 1110; CE Instruments, Milan, Italy). Samples were dried at 60°C for 24 h, and homogenized by mill grinding (Mixer Mill MM301; Retsch, Hann, Germany). Tissue phosphorous (P) was extracted using an alkaline persulfate digestion, and determined using a standard colorimetric phosphate protocol (Menzel and Corwin 1965).
Glucose content within the U. pertusa tissues was determined by using 3,5-dinitrosalicylic acid to measure reducing sugar (Wood and Bhat 1988); 70 mL of 0.1 M HCl was added to 3 g of dried sample in an Erlenmeyer flask, and reducing sugar was extracted by autoclaving at 121°C and 1.5 atm for 15 min.
Statistical analysis
All statistical analyses were performed using the SPSS 20.0 statistical software. Data normality and homogeneity of variance were determined using the Kolmogorov-Smirnov normality test and Levene’s homogeneity of variance test, respectively. One-way analysis of variance (ANOVA) and two-way ANOVAs were used to compare the effects of CO2 concentrations and temperatures on photosynthetic parameters, growth rate, Chl a concentration, and reducing sugar. Nonparametric tests (Kruskal-Wallis test) were conducted for data that were not normally distributed. When significant differences were detected, specific differences between treatment pairs were determined using Tukey’s multiple comparisons. A paired-sample t-test was used to aid in differentiating the eCA activity before and after the addition of eCA inhibitor.
RESULTS
Photosynthesis and Chl a fluorescence
Gross photosynthesis (GP) in U. pertusa determined from the photosynthesis-irradiance (P-E) curves did not vary significantly among the four CO2 and temperature treatments. Further, GP was saturated at 100–150 μmol photons m−2 s−1 and either decreased or reached a plateau over 150 μmol photons m−2 s−1 in all treatments (Fig. 1). However, GP in the acidification treatment showed distinctly higher photosynthesis and was 1.2–2 fold higher when the alga was exposed to over 150 μmol photons m−2 s−1 under all measured irradiances. The photosynthetic parameters obtained from the P-E curves are shown in Table 1. Maximum gross photosynthetic rates (GPmax) were greatest (1,046.9 ± 66.5 μmol O2 g−1 DW h−1) under acidification conditions, and lowest (622.9 ± 133.7 μmol O2 g−1 DW h−1) under warming conditions. There was a trend towards higher rates of photosynthesis in the elevated CO2 conditions (acidification and greenhouse; hereafter EC) (F = 6.986, p = 0.030), while the elevated temperature condition (warming and greenhouse; hereafter ET) negatively impacted GPmax (F = 6.366, p = 0.036). Given that increased photosynthesis under EC was offset by reduced photosynthetic rates under ET, GP of U. pertusa grown under greenhouse conditions did not exhibit any differences in GP relative to present conditions. There were also no differences in photosynthetic efficiency (α) and minimum saturation irradiance (Ek) among the treatments. Dark respiration (Rd) ranged between 68.6 and 252.7 μmol O2 g−1 DW h−1, with the highest Rd, observed under the present conditions nearly 2.5-fold higher than under the warming conditions. Consequently, Rd appeared to depend most upon temperature change, i.e., Rd of ambient temperature had twice as high as ET (F = 11.206, p = 0.010), while Rd did not appear to be impacted by EC conditions (p > 0.05).

Photosynthesis versus irradiance curves of Ulva pertusa under four CO2 and temperature conditions. PAR, photosynthetic active radiation, i.e., irradiance. Data are presented as mean ± standard deviation (n = 3).

Photosynthetic parameters of Ulva pertusa obtained from the four temperature and CO2 conditions (n = 3, mean ± SD)
The maximum quantum yields of PSII (Fv / Fm) ranged from 0.74 to 0.77, but there were no significant difference among the treatments (Table 2). However, there was a trend in which Fv / Fm was slightly decreased under ET conditions and increased under EC conditions, but again these patterns were no significant. Similarly, maximum electron transport rates (rETRm,RLC), electron transport efficiency (αRLC) and minimum saturation irradiance (Ek,RLC), as determined from the RLC, were also not significantly different among the treatments.
External CA activity
Gross photosynthesis (GP) in U. pertusa measured in the absence of AZ ranged from 386.5 to 876.3 μmol O2 g−1 DW h−1 when held at 70 μmol photons m−2 s−1 (Fig. 2). Indeed, GP was significantly inhibited (by 8–52%) in the presence of AZ (t = 5.180, p < 0.001). While U. pertusa possesses eCA activity as one of CCMs, this activity did not differ among the treatments. However, there was significant reduction (by 50%) in GP under the ambient CO2 conditions (present and warming) relative to EC conditions (F = 9.825, p = 0.017). Further, there were no significant impacts to photosynthesis caused by adding AZ under the different temperatures (p > 0.05), and not there were impacts of the combined effects of CO2 and temperature on eCA activity compared to the present condition.

Photosynthesis of Ulva pertusa under four CO2 and temperature conditions. Filled bars represent photosynthesis when acetazolamide (AZ) added. Data are presented as mean ± standard deviation (n = 3, *p < 0.05, **p < 0.01 for paired t-test between −AZ and +AZ and p > 0.05 for photosynthesis between the four conditions).
Chl a, tissue nutrient, and glucose contents
The trend for Chl a contents was similar to that for photosynthetic rate (Table 3). Specifically, there was no significant difference among the treatments, though a trend was observed in which, Chl a content was higher under EC conditions than under ambient CO2 conditions, but these differences were reduced with increasing temperature. Together, this indicates that Chl a contents in U. pertusa tissues were not different under greenhouse condition relative to present condition, suggesting that ET offsets the increased Chl a concentration that is induced by EC.
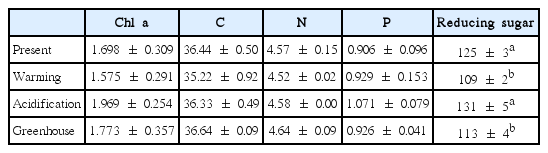
Chlorophyll a concentration (mg Chl a g−1 FW), tissue elemental contents (%) and glucose content (mg g−1 DW) of Ulva pertusa obtained from the four temperature and CO2 conditions (n = 3–5, mean ± SD)
The carbon and nitrogen contents in U. pertusa tissues were lower under ET conditions than in any of the other treatments (Table 3). Tissue P, in contrast, was highest under EC conditions and lowest under present conditions. However, individually and in combination, EC or ET levels significantly impacted neither elemental tissue contents or their ratios.
Glucose content within U. pertusa tissues was dependent on temperature (F = 66.589, p < 0.001) but not CO2 conditions. Specifically, glucose was significantly greater under EC conditions than present CO2 concentrations (F = 5.328, p = 0.05), while ET induced a significant decrease in the glucose content, by approximately 12% (Table 3).
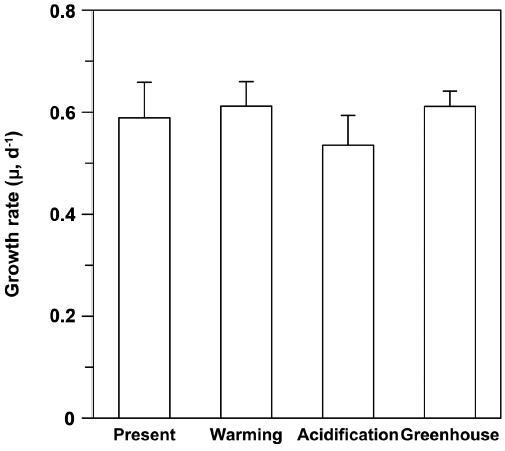
Growth rate
Growth rates (μ) ranged from 0.493 to 0.681 day−1, with the lowest μ occurring under EC conditions (Fig. 3). Although there were no significant differences among treatments, the μ trended higher under ET conditions, irrespective of CO2 levels (F = 4.319, p = 0.054), and it appeared that EC did not impact the growth rates of U. pertusa (p > 0.1). Lastly, the combined effect of CO2 and temperature did not elicit any difference on the growth rates.
DISCUSSION
It was shown previously that the growth rates of Ulva spp. were not significantly affected by enriched CO2 concentrations (Israel and Hophy 2002). The growth rate of U. pertusa in this study also did not differ between ambient and enhanced CO2 conditions, despite a slight upward trend in photosynthetic rates as CO2 levels rose (Figs 1 & 3). The growth of U. pertusa appears to be largely decoupled from photosynthesis; for example, photosynthetic rates were low under ET conditions, whereas the species growth rates were slightly higher under ET conditions (Figs 2 & 3). This finding indicates that growth is regulated not only by photosynthesis but also by other mechanisms, including respiration rate and release of organic C compounds that restrict the contribution of C fixation to growth (Davison 1991). The C balance of photosynthesis and respiration controls plant growth (Cheng et al. 2000). The growth rates of U. linza, which with their massive green tide floating in the Yellow Sea in early summer, were close to zero at higher temperatures despite having high level of photosynthesis, most likely due to high respiration and extensive fragmentation offsetting increasing rates of photosynthesis (Kim et al. 2011, Kang et al. 2014, 2016). In our study, the rate of photosynthesis was not significantly different among the treatments, but respiration decreased as temperature increased, and trends in growth rates corresponded to photosynthesis / respiration ratios (GP / Rd ratios) (data not shown). GP / Rd ratios ranged from 2.5 to 4.9 in the four treatments, with these ratios being slightly higher under ET conditions, but not impacted by CO2 concentration. Such high GP / Rd ratios suggest that photosynthates are used less for respiration and more for growth (Zou and Gao 2014). Indeed, Gordillo et al. (2001) showed a doubling in growth of U. rigida at high CO2 concentrations that were concurrent with a decline in organic carbon release when N was not limiting. Dissolved organic carbon (DOC) exudation, however, has been shown to increase in some eukaryotic phytoplankton under EC (Engel et al. 2005). Thus, it is possible that U. pertusa releases a large amount of DOC under EC, and this may affect its growth rate.
The highest growth rates of Ulva spp. have been observed at temperature between 10 and 20°C (Taylor et al. 2001), with various optimal temperatures for photosynthesis of U. pertusa reported for different locations: 15°C (Floreto et al. 1993), 20–25°C (Murase et al. 1993), and 20°C in Korean coastal waters (Kim et al. 2004). These differences are based on physiological variations among ecotypes adapted to particular geographic and seasonal conditions (Young et al. 1987, Schaum et al. 2013). In rocky shores of the south coast of Korea, for example, an intense bloom of U. pertusa often occurs during spring when seawater temperatures range between 12 and 18°C (Kim et al. 2004). Our experiment assumed 16°C to be the ambient temperature, with 19°C the elevated temperature condition, which correspond to the optimal temperature range for growth of Ulva species reported by Taylor et al. (2001). Further, our results show that while photosynthesis is negatively affected by high temperatures, growth is not. In general, high temperatures led to increased rates of respiration (Atkin and Tjoelker 2003), although we found that Rd was higher under ambient temperature conditions. The low Rd of U. pertusa under ET conditions reduces the loss of fixed C during dark periods, thereby increasing the fixed C used for growth (Davison et al. 1991), which may explain why there were no differences in growth rates among the four treatments.
Low eCA activity shows that organisms prefer CO2 or both HCO3− and CO2 as primary photosynthetic substrates (Mercado et al. 1997). U. pertusa exhibited significant eCA activity, but this activity did not change under EC and / or ET conditions (Fig. 2). This suggests that photosynthesis may be saturated under current oceanic DIC conditions and the species prefers to use CO2. This result is consistent with previous observations of CA activity in Ulva species (Björk et al. 1993), although some researches have shown that AZ may significantly inhibit photosynthesis in U. linza (Israel and Hophy 2002).
In this study, increased CO2 concentrations and temperatures did not enhance photosynthesis, growth rates or biochemical composition, with the exception of glucose content (Table 3). However, photosynthetic pigment composition did change with CO2 concentration in previous studies. Specifically, U. rigida grown under different CO2 conditions exhibits decreased pigment contents at high CO2 (Gordillo et al. 2003), and the photosynthetic pigments of Gracilaria tenuistipitata likewise decreases when DIC is enriched (5% CO2) (García-Sánchez et al. 1994). Further, Andria et al. (1999) showed similar results to those in our study in that DIC concentration did not impact the photosynthetic pigments of Gracilaria sp. In general, biochemical composition varies with nutrient conditions of the culture medium (Andria et al. 1999, Figueroa et al. 2009), and increasing CO2 concentrations enhance photosynthetic activity and increase nutrient uptake (Webber et al. 1994). Plants use tissue nutrients to supply the nutrient demand for photosynthesis, thereby nutrient limitation may occur under CO2 conditions. In this study, nutrients were replenished every 2 days, and as such nutrient limitation did not occur in any treatment, which may explain the absence of any significant differences in Chl a concentrations and / or tissue element contents. Glucose content, which is related to rates of photosynthesis and respiration, was lower under high as opposed to ambient temperatures. Carbohydrates storage is related to photosynthetic supply or respiratory demand (Falkowski and Raven 2007); given that, photosynthesis trended lower under ET condition than under present or EC conditions, possibly explaining why glucose production was reduced in this study. Simultaneously, respiration was also reduced, and this might be related to the need to maintain carbon balance at lower glucose levels.
It is often argued that global climate change causes more frequent harmful algal blooms (Dale et al. 2006) but our results suggest that this may not be the case with U. pertusa. Increased temperatures above typical temperatures in the spring when the bloom naturally occurs do not appear to increase U. pertusa growth rates. We suggest that U. pertusa would not favor the rising temperatures associated with global climate change, and it is unlikely that the blooms would be shifted to winter because of light limitations on photosynthesis. Furthermore, higher CO2 levels did not significantly increase growth or photosynthesis. It could be that blooms of U. pertusa in Korean waters may not, in fact, change because of constraints by temperature. This does not preclude the possibility that other algal species with naturally occurring higher temperature requirements may be a source of blooms, especially since there is no prospect for regional declines in nutrient inputs and the resulting eutrophication of nearshore coastal environments. Increased temperature not only affects organisms directly but also influences the transport of nutrients from land-based sources to aquatic environments. Thus, future studies should combine climate change and other factors like light, nutrient loads, and salinity, given that many such factors will be altered by future CO2 concentrations and consequent temperature increases. For instance, photosynthetic responses related to temperature and CO2 depend on light availability (Kim et al. 2013), and net photosynthetic rate decreases with increasing temperatures at subsaturating light levels, whereas the light compensation point increases as temperature rise (Davison 1991).
In recent years, research has focused on the response of whole communities to increased CO2 and temperature. Rodolfo-Metalpa et al. (2011), for example, showed that the rising temperature could aggravate the benthic communities combined with ocean acidification in the Mediterranean Sea. In the case of the phytobenthos communities, the effects of rising CO2 levels singularly or combination with higher temperatures induced a variety of responses from individual or assemblages of macroalgal species, and high CO2 and high temperature were shown to reduce macroalgal assemblage biomass (Olabarria et al. 2013). There may also be a synergistic positive effect of high CO2 and high temperature on algal turfs, but changes in the algal turfs are also exacerbated kelp loss (Connell and Russell 2010). As mentioned above, the combined effects of CO2 and temperature vary and are complex at both the individual and community levels. Our research is relevant to understanding the changes in photosynthetic characteristics and growth responses of a coastal bloom-forming algal species with respect to future climate change as represented by ocean acidification and global warming.
ACKNOWLEDGEMENTS
We would like to thank Dr. J.-H. Kim for technical support and comments on the manuscript. This research was supported by the program on “Management of marine organisms causing ecological disturbance and harmful effects,” which was funded by KIMST/MOF.