Antibacterial compounds in green microalgae from extreme environments: a review
Article information
Abstract
Increased proliferation of bacterial resistance to antibiotics is a critical issue that has increased the demand for novel antibacterial compounds. Antibacterial activities have been evaluated in extracts from photosynthetic green microalgae, with varying levels of subsequent potential for development based on the strain of algae, strain of bacterial pathogen, and solvent used to extract the metabolites. Green microalgae from extreme environmental conditions have had to adapt to conditions that exclude many other organisms. The production of antibacterial compounds aids directly or indirectly in the survival of green microalgae in these extreme environments, as well as potentially serve other roles. This review investigates antibacterial activities of green microalgae from both extreme in-situ environmental conditions and induced extreme laboratory conditions and highlights.
INTRODUCTION
Microalgal secondary metabolites are a diverse group of molecules that are not necessary for basic cell functioning and are known to exhibit other important biological properties including antibacterial activity (Lustigman 1988, Das and Pradhan 2010, Pradhan et al. 2014). In general, secondary metabolites seem to aid cells in their interactions with their environment, including signaling other organisms and protecting themselves against predators and competitors as well as from abiotic stress (Lustigman 1988, Leflaive and Ten-Hage 2007, Challouf et al. 2012, Senhorinho et al. 2018). Stress can, therefore, play an important role on microalgal production of antibacterial compounds. For example, strains of Dunaliella salina collected from waters contaminated with human sewage and industrial wastes produced more compounds displaying antibiotic activity than strains from low pollution areas (Lustigman 1988). Secondary metabolites have been a valuable source in the development of new pharmaceuticals, such as antibiotic, anti-inflammatory and anti-cancer drugs (Namdeo 2007).
Green microalgae are eukaryotic organisms able to survive in extreme environments in which other eukaryotic planktonic algae cannot (Fogg 2001). Their cell processes require these microorganisms to maintain a constant intracellular environment by adapting to external changes, an adaptation that can cause an increase in energy consumption leading to a decrease in the photosynthetic metabolism (Gerloff-Elias et al. 2005). This decrease can result in an accumulation of intermediate compounds within the cells, which are acted upon by subsequent pathways to form secondary metabolites (Malik 1980). As a consequence, there have been a number of studies focused on investigating antibacterial activity from green microalgae isolated from extreme environments, with the hope to find unique and effective compounds (Challouf et al. 2012, Giddings and Newman 2015a, Navarro et al. 2017, Senhorinho et al. 2018, Santhakumaran et al. 2020a).
Compounds originated from microalgae exhibiting antibacterial activity include fatty acids, glycolipids, phenolics, terpenes, β-diketone, and indole alkaloids (Ördög et al. 2004). However, most antibacterial activity is usually attributed to long chain unsaturated fatty acids (Borowitzka 1995). The interest in microalgae as a source of antibiotics seems to have originated with the work of Pratt et al. (1944). They investigated Chlorella, a genus of freshwater green microalgae capable of producing chlorellin, an antibacterial compound able to inhibit the growth of both Gram-positive and Gram-negative bacteria. Since then, additional studies have shown that some green microalgae produce substances that can kill or inhibit the growth of human pathogens (Das and Pradhan 2010, Challouf et al. 2012, Elkomy et al. 2015, Mezzari et al. 2017, Navarro et al. 2017).
Previous reviews have mainly focused on antibacterial activity of microalgae, including cyanobacteria and eukaryotic microalgae from non-stressed environments (Pradhan et al. 2014, Senhorinho et al. 2015, Shannon and Abu-Ghannam 2016). This review focuses on green microalgae from extreme environments that exhibit antibacterial activity against human pathogens. This includes the range of extreme environments, the spectrum of purported antibacterial compounds, the specific environmental conditions that may impact compound formation, and how effective the compounds are.
SCREENING FOR ANTIBACTERIAL COMPOUNDS FROM GREEN MICROALGAE
The increased availability of technologies to detect, purify, isolate and identify compounds present in complex extracts, has allowed researchers to pursue the challenging path of natural product screening (Senhorinho et al. 2015). The steps for screening microalgal extracts usually include selecting organic solvents and extraction method, testing methodology for antibacterial activity, and eventually fractionating extracts as an attempt to isolate the active compounds (Ohta et al. 1995, Navarro et al. 2017). In studies involving green microalgae from extreme environments, traditional solvent extraction or solid-liquid extraction is usually performed, in which cells are extracted by Soxhlet extractor, maceration or sonication with a series of solvents with increasing polarity to ensure recovery of different microalgal products (Al-Wathnani et al. 2012, Challouf et al. 2012, Corona et al. 2017, Navarro et al. 2017, Jafari et al. 2018, Kilic et al. 2018). The Soxhlet extraction, or continuous hot extraction, is simple and inexpensive, does not require filtration after extraction, allows the extraction of many different compounds and it permits simultaneous extraction. However, compounds can be decomposed due to temperature and long extraction time, and a large amount of solvent are required (Zygler et al. 2012). Maceration with continuous stir is another common approach to obtain antibacterial compounds from microalgae. It is inexpensive and easy to perform, and it allows the extraction of non-heat stable compounds, but it has the disadvantages of requiring long extraction time, and filtration or centrifugation followed by evaporation of solvent after extraction (Abubakar and Haque 2020). In sonication, or ultrasound-assisted extraction, high frequency energy (higher than 20 kHz) is applied to microalgal cells allowing solvent penetration (Abubakar and Haque 2020). It has the advantages of reducing extraction time and requiring smaller amounts of solvents, which allow for the extraction of small amounts of microalgal biomass. However, its main drawbacks include the loss of compounds due to high energy degradation and the difficulty of reproduction (Abubakar and Haque 2020).
When the extract is obtained, a standardized in vitro bioassay is usually chosen, following the guidelines from the Clinical and Laboratory Standards Institute or from the European Committee on Antimicrobial Susceptibility Testing (EUCAST), including agar diffusion and / or broth dilution techniques (Challouf et al. 2012, Cakmak et al. 2014, Alwathnani and Perveen 2017, Corona et al. 2017, Navarro et al. 2017, Jafari et al. 2018, Kilic et al. 2018, Senhorinho et al. 2018). Once promising activity is detected, the extract is fractionated, and bioactive assays performed at each level of purification in order to isolate the active compounds (Beutler 2019). However, to date, separation of compounds from green microalgae obtained from extreme environments has not been commonly performed. The few studies carrying out further analysis of microalgal extracts used methods such as column chromatography, gas chromatography and high-performance liquid chromatography, as well as hyphenated techniques such as gas chromatography-mass spectrometry (Ohta et al. 1995, Navarro et al. 2017).
Most microalgal compounds exhibiting antibacterial activity have not been identified. The known antibacterial compounds from microalgae isolated from extreme environments include saturated and unsaturated fatty acids such as palmitic acid, linoleic acid, linolenic acid and oleic acid. Lipid extraction from microalgal cells is usually performed using non-polar solvents such as chloroform, hexane or dichloromethane, or solvent mixtures such as chloroform-methanol and hexane-isopropanol (Barkia et al. 2019). Techniques used to obtain active extracts / compounds are listed in Table 1.
GREEN MICROALGAE GROWING IN STRESSED ENVIRONMENTS
Microalgae can be found in a wide range of stressed or extreme environments, such as fresh or salt water bodies with low pH and / or municipal wastewater contamination (Giddings and Newman 2015b). Whilst investigation of secondary metabolites from green microalgae has grown since the work of Pratt et al. (1944), these have been less explored, and only recently become the focus of attention (Taş et al. 2015, Alwathnani and Perveen 2017, Senhorinho et al. 2018).
The environments classified as extreme are those with non-circumneutral pH, high temperatures, high dissolved solutes potentially including metals, organic-rich wastewater (e.g., from municipal waste effluents), and arid / desert environments, which have been summarized in Table 2. The microalgae found in these environments are currently underrepresented as cultured type strains, and often must be first collected and isolated, which adds an additional level of difficulty in contrast to testing existing isolates established in culture collections, which are more typically from mesophilic environments. Since novel strains from extreme environments have not been widely explored, their investigation should increase the chances of finding species with unique metabolites and capabilities (Senhorinho et al. 2018).
Mining impacted environments
Low pH
Green microalgae obtained from mining-impacted water bodies with pH lower than 3 have been shown to produce antibacterial compounds capable of inhibiting the growth of both Gram-negative and Gram-positive bacteria (Navarro et al. 2017, Senhorinho et al. 2018). In order for green microalgae to survive in low pH waters they have to maintain a circumneutral intracellular pH (Gimmler 2001, Gerloff-Elias et al. 2005). To achieve this, high amounts of H+ ions need to be prevented from entering the cell, which can also restrict the access of other important ions that are necessary for survival, such as K+ (Fogg 2001). Although not well understood, this increased ionic stress seems to influence the production of secondary metabolites by microorganisms (Malik 1980), which may indirectly be related to antibacterial activity.
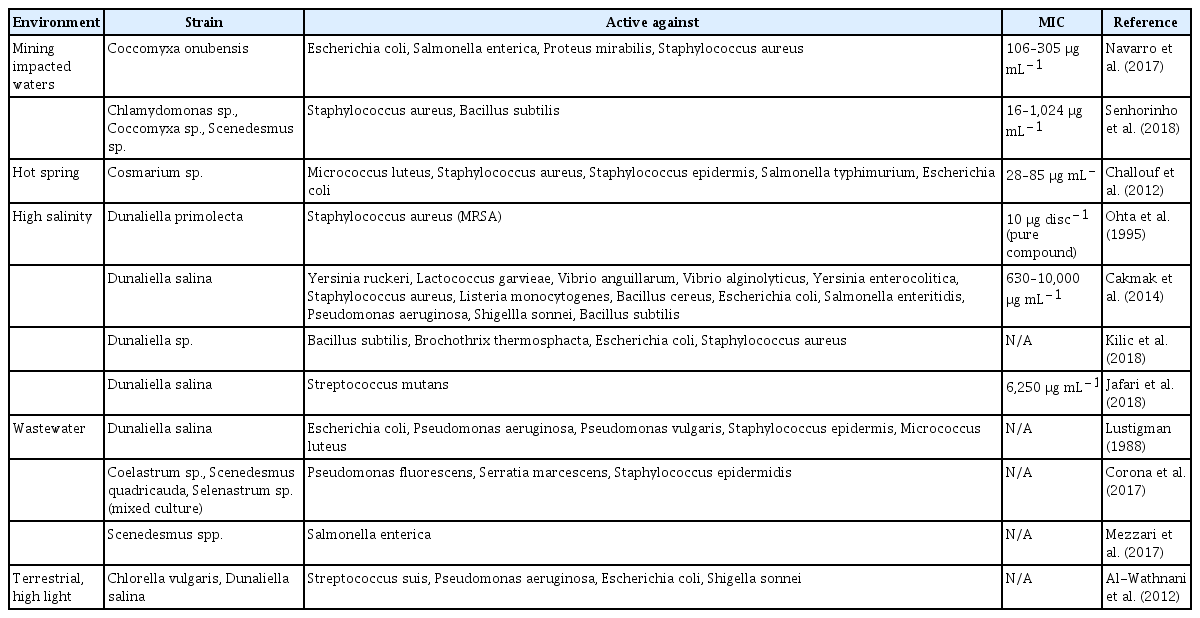
Although studies investigating antibacterial activity of green microalgae induced at low pH have not yet identified specific compounds, crude extracts from these microorganisms have shown promising activity suggesting these extreme environments as potential sources of strains that should be further investigated. According to Navarro et al. (2017), intracellular extracts from the acidophilic (pH 2.5–4.5) green microalga, Coccomyxa onubensis obtained using either hexane, diethyl ether, chloroform or dichloromethane, exhibited antibacterial activity, particularly against Gram-negative bacteria. The green microalgae were grown at the original pH of 2.5 throughout the experiments and the results from minimum inhibitory concentration (MIC) assays ranged from 106–305 μg mL−1. It was suggested that fatty acids could be involved in the activity due to increased activity from extracts obtained with non-polar solvents compared to polar solvents. The presence of fatty acids was confirmed using gas chromatography (Navarro et al. 2017). As no pure compounds were tested and the active compounds are likely to be found only in small concentrations within the cell, further fractionation to determine what compounds were responsible for the activity should also decrease the MIC (Navarro et al. 2017).
High iron levels
Mining impacted areas often have increased levels of metals in the soil and water surrounding them, including an increased level of iron, copper and nickel (Cummings et al. 2000). Microalgae growing in areas with metal concentrations higher than needed for cell growth, may be impacted by oxidative stress leading to changes in metabolism (Hu 2013).
Antibacterial activity was observed from green microalgae bioprospected from water bodies (pH 2.9–8.4) near abandoned mines with high metal concentrations (Senhorinho et al. 2018). Levels of iron were up to 22 ppm, compared to water guidelines for protection of aquatic life that indicate the acceptable limits as less than 0.2 ppm (Hem 1972). Their results indicated that green microalgae, particularly Chlamydomonas spp., were active against the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis. The extracts obtained through the use of methanol exhibited some very low MIC values, such as 16 μg mL−1 against S. aureus. Extracts exhibiting low MIC should be further fractionated to determine what chemical groups are associated with the activity and to determine if the MIC lowers even further after fractionation.
Extreme temperatures
Most commonly, high microalgal growth rates are associated with 20–30°C, but microalgae have been found in a wide range of temperatures, from Antarctic polar waters to geothermal springs reaching 70°C (Costas et al. 2008, Xin et al. 2011). The temperature for optimum growth rate and maximum production of antibacterial compounds by Chlorella marina was found to be 25°C compared to the other tested temperatures between 20–40°C (Elkomy et al. 2015). However, other studies investigating antibacterial activity from microalgae have shown that the optimum growth temperature does not necessarily equate to the highest production of antibacterial compounds, as the enhanced production of secondary metabolites can be independent of optimum level of growth (Schuelter et al. 2019).
Geothermal springs typically have temperatures between 40 and 70°C (Costas et al. 2008), and the ability for microalgae to survive in this temperature range is thought to be due to spontaneous genetic mutation (Costas et al. 2008). In general, when temperature is increased green microalgae have been found to have an overall decrease in lipids, including polyunsaturated fatty acids, which have been associated with antibacterial activity (Hu 2013).
Cosmarium sp. collected from a hot spring (60°C) in Tunisia exhibited antibacterial activity against Gram-positive and Gram-negative bacteria (Challouf et al. 2012). Since solvents with different polarities (hexane, acetone, or water) were used to obtain the crude extracts, it is likely that different compounds exhibited antibacterial effects that resulted in the production of MIC below 100 μg mL−1 for all susceptible bacteria (Challouf et al. 2012). Whereas, a previous study looking at Cosmarium laeve did not find significant antibacterial activity when the microalga was collected from a non-extreme river environment (Abdo et al. 2012). What is not known is the impact of different temperatures for the extremophilic Cosmarium sp. on antibacterial activity or what chemical compounds are directly involved in the activity, which should indicate the importance of green microalgae as antibacterial producers (Forján et al. 2015).
Hypersaline environments
Marine environments typically have a salinity percentage around 3.5% (35,000 mg L−1), but there are inland lakes that have salinity percentages reaching 35% (350,000 mg L−1), where some halophilic species of green microalgae such as D. salina have been found (Cakmak et al. 2014). Microalgae from areas of high salinity, such as Dunaliella spp., and Chlamydomonas nivalis, have shown increased lipid content, for instance, whereas in others high salinity environments led to a reduced lipid content in species such as Botryococcus braunii (Hu 2013).
Dunaliella is a genus of green microalgae that thrives in high salinity environments, and is the only group of well-known eukaryotic photosynthetic organisms capable of surviving in 45% salinity (450,000 mg L−1) (Cakmak et al. 2014, Kilic et al. 2018). This genus of green algae is currently used in the production of the pigment β-carotene for food colouring and for the antioxidant properties it possesses (Burton and Ingold 1984). In high salinity, Dunaliella has been found to increase its production of certain secondary metabolites, such as β-carotene, by up to 14%, which may suggest an ability to produce metabolites with antibacterial activity (Cakmak et al. 2014). In all four studies listed in Table 2, except for Kilic et al. (2018), Dunaliella species were cultured in a standard marine medium where multiple salt concentrations were tested to determine the effect that salinity would have on antibacterial production (Ohta et al. 1994, Cakmak et al. 2014, Jafari et al. 2018). Increasing salinity percentage from 10 to 20% was shown to increase antibacterial activity of the chloroform extract using a disc diffusion assay (Kilic et al. 2018). However, in another study where salinity was not maintained at a high concentration during growth, the extracts obtained from D. salina inhibited Gram-positive and Gram-negative bacteria only at relatively high concentrations (MIC 630–10,000 μg mL−1) (Cakmak et al. 2014, Jafari et al. 2018). These high values may be associated to either low concentrations of antibacterial compound(s) within the crude extract, or the release of the antibacterial compounds to the culture medium, as observed in previous reports in which Dunaliella showed increased production of secondary metabolites in hypersaline environments (Cakmak et al. 2014, Jafari et al. 2018). By looking at the antibacterial activity of unsaturated and saturated fatty acids from Dunaliella primolecta extracts, Ohta et al. (1994) determined that γ-linolenic acid exhibited the highest activity against S. aureus (10 μg per disc).
Wastewater
Green microalgae have been used to mitigate contaminants from wastewater and excess nutrients that result from the disposal of human and animal waste (Zhou et al. 2014). Algae that are naturally found in areas with high levels of industrial or human waste, or those that are added in order to further help with decontamination, are likely to have to cope with high levels of resource competition from other members of the microbial community. Studies have suggested that these contaminated environments could potentially encourage microalgal cells to produce antibacterial compounds (Zhou et al. 2014, Mezzari et al. 2017).
Butanol extracts from D. salina bioprospected from waters that had been polluted with industrial waste and sewage demonstrated higher level of antibacterial activity compared to strains from uncontaminated waters (Lustigman 1988). The strains of bacteria that were susceptible to the extracts included Escherichia coli and Proteus vulgaris, which are commonly found in fecal matter (Lustigman 1988). Rather than using extracts, whole cells of Scenedesmus spp., isolated from swine wastewater were able to eliminate Salmonella enterica growth within 48 h (Mezzari et al. 2017). However it was unclear in this study whether the activity was due to secondary metabolites produced by the green microalgae, or the high pH 11 that was reached in the water during photosynthetic activity (Mezzari et al. 2017).
Polyunsaturated aldehydes from Coelastrum sp., Scenedesmus quadricauda, and Selenastrum sp. obtained from a wastewater treatment plant inhibited the Gram-negative bacterium Serratia marcescens in a disc diffusion assay (Corona et al. 2017). This study specifically targeted the extraction of polyunsaturated aldehydes, which are a product of the degradation of free polyunsaturated fatty acids after cell integrity is compromised (Ribalet et al. 2008). Polyunsaturated aldehydes have been shown to not only possess antibacterial activity, but also reduce the cell proliferation of human cancer cell lines, and reduce the growth of fungi (Ribalet et al. 2008). The cascade known to produce the polyunsaturated aldehydes is well documented in diatoms, but there are few studies specifically investigating this activity in green microalgae (Ribalet et al. 2008, Vidoudez and Pohnert 2008).
Terrestrial environments
Green microalgae are commonly found in aquatic habitats, but some species are also capable of surviving in terrestrial environments, and under extreme conditions (Rindi et al. 2011). Green microalgae are commonly found in soil where they contribute to nutrient cycling and uptake of certain heavy metals, such as cadmium, zinc and copper (Yoshida et al. 2006). Green microalgae living near the surface of soils, on rocks or other terrestrial environments need to mitigate the effects of UV-B radiation, which are especially high in desert environments. Using mycosporine-like amino acids (MAA), a group of secondary metabolites, as a protective mechanism, both aquatic and terrestrial green microalgae have been able to endure high amounts of UV-B radiation (Xiong et al. 1999). Desert environments increase the production of MAAs as a response to the increased light, and, therefore, may also induce metabolic changes associated with antibacterial activity (Xiong et al. 1999).
There have not been many studies investigating green microalgae from extreme terrestrial environments. Al-Wathnani and Perveen (2017) did evaluate Chlorella vulgaris and D. salina from desert soils for antibacterial activity. The results indicated that extracts obtained with methanol : acetone : diethyl ether (5 : 3 : 1 volumes) were able to inhibit the growth of both Gram-negative and Gram-positive bacteria (Alwathnani and Perveen 2017). In an attempt to determine the active organic compounds, mass spectrometry was performed. However, without testing the active fractions through MIC test, it cannot be definitively concluded that the most concentrated components were also the most active against the bacterial strains (Alwathnani and Perveen 2017).
MODIFIED CULTURE CONDITIONS
Studies on modifying the culture conditions of green microalgae have shown differences in antibacterial activity (Elkomy et al. 2015, Dineshkumar et al. 2017, Hamouda and Abou-El-Souod 2018). Similar to bacteria and fungi (Lo Grasso et al. 2016), it is suggested that by changing conditions to induce cell stress, microalgae may be stimulated to produce secondary metabolites with antibacterial activity, as well as potentially larger quantities of these secondary metabolites (Ruffell et al. 2016). Culture condition modifications include media composition, pH, light, and temperature (Schuelter et al. 2019, Santhakumaran et al. 2020b).
The media typically used to grow green microalgae in the laboratory are either Bold’s Basal Medium (BBM) or Blue-Green Medium (BG-11), both of which contain generally the same nutrient compounds but at different concentrations, including N, P, K, Mg, Ca, S, Fe, Cu, Mn, and Zn. The main components that have been modified to stimulate production of antibacterial compounds are the main salts, including MgSO4, CaCl2, K2HPO4, NaCl, NaNO3, and EDTA (ethylenediaminetetraacetic acid) (Ohta et al. 1995, Hamouda and Abou-El-Souod 2018). The required concentrations of these medium components, including the macronutrient salts, can be species or even strain specific (Grobbelaar 2013, Procházková et al. 2014). Each of the macronutrients plays a large role in primary metabolism, with for example, a decrease in nitrogen concentration causing a decrease in cell chlorophyll concentration, thereby altering colour and eventually the accumulation of oils (Grobbelaar 2013). By altering the primary metabolism of green microalgae, it is possible for different secondary metabolites to be produced and hence a change in antibacterial activity.
Various concentrations of MgSO4, CaCl2, K2HPO4, NaCl, NaNO3, and EDTA in microalgal medium were tested in order to determine which concentrations would stimulate higher antibacterial activity in green microalgae (Ohta et al. 1995). Each media component was tested individually, finding improved antibiotic production when concentrations of MgSO4 were increased from the 0.6 mM of standard medium to 12 mM, as well as increasing phosphate from 0.3 to 3 mM, and decreasing CaCl2 from 30 to 3 μM (Ohta et al. 1995). Methanolic extract from green microalgae grown in the new media yielded higher anti-MRSA (methicillin resistant Staphylococcus aureus) activity, which suggested higher levels of antibiotic production. There was over a two fold increase of antibiotic production, but a very small increase in biomass, which indicated that the increase in antibiotic activity was not caused by higher quantities of algal cells alone (Ohta et al. 1995). The increase in antibiotic production allowed for purification and identification of substances responsible for the anti-MRSA activity, which were unsaturated fatty acids, with linolenic acid yielding the highest activity (Ohta et al. 1995).
Phosphate is considered growth limiting as it often precipitates and is unavailable for uptake by microalgae (Grobbelaar 2013). However, it was observed that by increasing the concentration of phosphate past the usable threshold it can induce stress via toxicity and, therefore, alter metabolism (Grobbelaar 2013). When sulphate is limited in the media, microalgal cells can reduce the rate of both photosynthesis and protein synthesis, and also affect the ratio of carotenoids to chlorophyll pigments within the cell (Procházková et al. 2014). The effect of changes in the concentration of phosphorus available to green microalgae on their antibacterial activity has been investigated (Hamouda and Abou-El-Souod 2018). Phosphorus is pivotal to lipid accumulation and usually added to the medium in the form of K2HPO4. Different concentrations of phosphorus (0, 0.0035, 0.007, 0.01, and 0.014 g L−1) were investigated, with the highest growth rate of S. obliquus being reached at 0.007 g L−1 of phosphorus, and the highest level of activity of the microalgal methanolic extract against S. aureus observed at 0.01 g L−1 of phosphorus (Hamouda and Abou-El-Souod 2018).
Untreated municipal wastewater, a nutrient rich medium, was used to determine if an increase in algal biomass or antibacterial compound production would be achieved (Dineshkumar et al. 2017). When C. vulgaris was allowed to grow in either BBM medium or in the wastewater medium, the biomass levels from wastewater were higher after 28 days (0.402 g L−1) compared to BBM (0.268 g L−1). Methanolic extracts from the cells grown in wastewater inhibited the growth of all bacterial species tested (Klebsiella pneumoniae, Proteus mirabilis, Vibrio chlorerae, Salmonella Typhi, E. coli, S. aureus, B. subtilis, Enterococcus sp., C. botulini, and Nocardia sp.) (Dineshkumar et al. 2017). However, unfortunately there was no comparative analysis on the activity of the microalgae grown in BBM versus the wastewater medium.
Of the culture conditions that have been explored, light has been the most investigated (Schuelter et al. 2019). The investigation of the effect of light on microalgal cells started when Pulich (1974) noticed that a strain of Chlorella sorokiniana was resistant to UV damage, even though other strains of the same species had a low survival rate (Pulich 1974). This suggested the potential for green microalgae to develop photoprotection in order to survive. Under low light conditions the amounts of chlorophyll a, b, and c, and phycobiliproteins will increase in order to capture more light to maintain cellular functions. However, when light intensity is high the opposite is true, with light harvesting pigments being reduced and protective carotenoids being increased (Hu 2013). Recently, the effect of changes in light on antibacterial agent production by green microalgae has been investigated (Elkomy et al. 2015, Kilic et al. 2018, Schuelter et al. 2019). Chlorella marina under different light intensity (1,000, 2,000, and 3,000 lux) exhibited different antibacterial activities, with cells producing more biomass under the highest light intensity (3,000 lux) and also being the most active at inhibiting bacterial growth (S. aureus and Serratia marcescens) (Elkomy et al. 2015). Extracts from Dunaliella sp. grown at 3,600 lux yielded the highest activity against E. coli (a difference of 4 mm in the disc diffusion assay), but when screened against B. subtilis, those obtained from exposure to 2,400 lux yielded the highest activity (a difference of 2 mm in the disc diffusion assay) (Kilic et al. 2018). This study suggests, therefore, that the level of antibacterial activity can vary between depending on the intensity of light applied and species of bacteria screened. However, the study went onto find that in general the highest light intensity (4,800 lux) exposure yielded the overall highest level of antibacterial activity, suggesting that high light exposure is more likely to induce the production of antibacterial compounds by Dunaliella sp. (Kilic et al. 2018). Furthermore, as the chlorophyll content of the cells decreased with increasing light intensity, photoprotective compounds such as phenolics which have a role in antibacterial activity may have been produced (Kilic et al. 2018). The production of photoprotective compounds is necessary in order to enhance survival under the intense light conditions and avoid cellular damage (Fujita et al. 2001). The necessary alteration in metabolism, therefore, produce secondary metabolites which may have a dual function of photoprotection and antibacterial activity (Hu 2013, Kilic et al. 2018).
Chlorophyll a absorbs light in two major areas within the visible spectrum, between 450–475 nm in the blue range and between 630–675 nm in the red range (Masojídek et al. 2013). In order to optimize photosynthesis and microalgal growth, a combination of red and blue bands of light are commonly used to maximize the energy the cells are able to directly utilize through chlorophyll a (Baba et al. 2012). When white light is used to grow algae it is exposed to all wavelengths in the visible spectrum and, however, not all of the light can be absorbed by chlorophyll a, and other photopigments are utilized to absorb the remaining light (Masojídek et al. 2013). Therefore, rather than investigating light intensity, the study by Schuelter et al. (2019) investigated the effect that a change in light source would have on antibacterial activity. By investigating the colour of light used to grow different isolates of green microalgae, it was theorized that different photoreceptors could be utilized compared to traditional blue and red bands known for aiding in photosynthesis (Schuelter et al. 2019). Using the green bands of light from light emitting diode (LED)-based bulbs to grow the samples yielded the highest level of antibacterial activity with inhibition halos greater than 20 mm, compared to red, blue or white LED or fluorescent light, which had inhibition halo means of 15 mm (Schuelter et al. 2019). Changes in green microalgae based on the colour of light have been noted to induce changes in the cell’s interaction with the environment and cellular functions (Cepák and Přibyl 2006).
CONCLUSIONS AND FUTURE DIRECTIONS
Green microalgae from stressed environments are capable of producing compounds with antibacterial activity (Challouf et al. 2012, Najdenski et al. 2013, Senhorinho et al. 2018). Many studies isolated microalgae from extreme environments, but grew the cultures using standard media and laboratory conditions. The culture environment can be artificially manipulated in order to allow for an increased production of antibacterial agents (Ohta et al. 1995, Hamouda and Abou-El-Souod 2018). An interesting approach only utilized in the study by Navarro et al. (2017), was to attempt to mimic aspects of the stressful environment while growing green microalgae in laboratory. This approach can result in a more realistic potential for antibacterial production and allow researchers to manipulate the culture conditions in order to stimulate microalgae to increase the production of active compounds.
A main challenge found in the studies investigating antibacterial activity of green microalgae is the limited number that use the MIC quantitative method (Weinstein and Lewis 2020). This method not only allows comparisons between extracted compounds from different strains and environments, but also provides quantitative assessment of effectiveness in comparison to available antibiotics. While variances in protocols are to be expected, the determination of the MIC of compounds / extracts by all studies would certainly increase the ability of researchers to directly compare their findings. Moreover, even in studies that have the MIC determined, this is usually where the research is stopped, with no further investigation into fractions of the crude extract to determine the active compounds.
Further investigations should attempt to guide the medium and culture conditions that the bioprospected species of green microalgae were originally found, in order to preserve the mechanisms that may be responsible for the production of secondary metabolites with antibacterial activity. Fractionation and identification of the active bioactive compound(s) should be carried out, as this is continuously highlighted as the goal of studies investigating antibacterial activity (Ohta et al. 1994, 1995). Following the initial screening and determination of the minimum inhibitory concentration, non-target toxicity studies should also be performed in order to confirm the possibility of the use of the secondary metabolites as a marketable product in order to address the shortage of novel antibacterial drugs.
Green microalgae have proven to be a potential new source of antibacterial agents, and the continued investigation of strains originating from stressed environments should allow for new strains with such potential to be discovered.
ACKNOWLEDGEMENTS
The authors would like to thank OCE (Ontario Centres of Excellence) and Mitacs Canada for their support.
Notes
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.