Interactions between common heterotrophic protists and the dinoflagellate Tripos furca: implication on the long duration of its red tides in the South Sea of Korea in 2020
Article information
Abstract
The mixotrophic dinoflagellate Tripos furca causes red tides in the waters of many countries. To understand its population dynamics, mortality due to predation as well as growth rate should be assessed. Prior to the present study, the heterotrophic dinoflagellates Noctiluca scintillans, Polykrikos kofoidii, Protoperidinium steinii, and mixotrophic dinoflagellate Fragilidium subglobosum were known to ingest T. furca. However, if other common heterotrophic protists are able to feed on T. furca has not been tested. We explored interactions between T. furca and nine heterotrophic dinoflagellates and one naked ciliate. Furthermore, we investigated the abundance of T. furca and common heterotrophic protists in coastal-offshore waters off Yeosu, southern Korea, on Jul 31, 2020, during its red tide. Among the tested heterotrophic protists, the heterotrophic dinoflagellates Aduncodinium glandula, Luciella masanensis, and Pfiesteria piscicida were able to feed on T. furca. However, the heterotrophic dinoflagellates Gyrodiniellum shiwhaense, Gyrodinium dominans, Gyrodinium jinhaense, Gyrodinium moestrupii, Oblea rotunda, Oxyrrhis marina, and the naked ciliate Rimostrombidium sp. were unable to feed on it. However, T. furca did not support the growth of A. glandula, L. masanensis, or P. piscicida. Red tides dominated by T. furca prevailed in the South Sea of Korea from Jun 30 to Sep 5, 2020. The maximum abundance of heterotrophic dinoflagellates in the waters off Yeosu on Jul 31, 2020, was as low as 5.0 cells mL−1, and A. glandula, L. masanensis, and P. piscicida were not detected. Furthermore, the abundances of the known predators F. subglobosum, N. scintillans, P. kofoidii, and Protoperidinium spp. were very low or negligible. Therefore, no or low abundance of effective predators might be partially responsible for the long duration of the T. furca red tides in the South Sea of Korea in 2020.
INTRODUCTION
Red tides, which are discolorations due to plankton blooms, often cause large-scale morbidity and mortality of marine organisms such as fish, sea birds, and marine mammals (Landsberg 2002, Shumway et al. 2003, Flewelling et al. 2005, Jessup et al. 2009), as well as economic losses to aquaculture and tourism industries (Shumway 1990, Anderson 1997, Azanza et al. 2005, Jeong et al. 2021). To minimize financial losses, it is critical to understand and forecast the outbreak, persistence, and decline of red tides (Jeong et al. 2015). In models for predicting red tide dynamics, growth and mortality due to predation are the most important parameters.
The cosmopolitan dinoflagellate Tripos furca is renowned for causing red tides across the waters of many countries (Dodge and Marshall 1994, Horner et al. 1997, Tomas 1997). This dinoflagellate can grow autotrophically or mixotrophically (Smalley et al. 1999, 2003, Smalley and Coats 2002, Baek et al. 2008). Tripos furca is known to prey on the choreotrich ciliates Strobilidium spp., small tintinnids with sizes of 10–40 μm in diameter, and the mixotrophic ciliate Mesodinium rubrum (Bockstahler and Coats 1993, Smalley et al. 1999, Stoecker 1999, Johnson et al. 2013). Meanwhile, the heterotrophic dinoflagellates Noctiluca scintillans, Polykrikos kofoidii, Protoperidinium steinii, and mixotrophic dinoflagellate Fragilidium subglobosum are known to feed on this species (Hansen and Nielsen 1997, Jeong et al. 2001, Olseng et al. 2002, Drits et al. 2013). However, predation by many other common heterotrophic protists on T. furca has not been explored. To understand its red tide dynamics, feeding by common heterotrophic protists on T. furca should be investigated.
Tripos furca is one of the dinoflagellates that frequently cause red tides in Korean waters (Baek et al. 2011, Lee et al. 2013, Jeong et al. 2017). It usually causes red tides during spring and summer around the Korean coast, mainly at 24.6–25.1°C, although it has been recorded as being abundant at 17.5–28.4°C (Jeong et al. 2013). This species caused long-lasting red tides in the South Sea of Korea from Jun 30 to Sep 5, 2020 (NFRDI data; http://www.nifs.go.kr/portal/redtideInfo). It is worthwhile to explore the roles of potential predators in the long duration of T. furca red tides because high grazing impact by heterotrophic protists sometimes causes cessation of red tides (Watras et al. 1985, Nakamura et al. 1992, Jeong and Latz 1994, Turner 2006, Stoecker et al. 2008, Yoo et al. 2013, Yang et al. 2019). If there was a shortage of effective predators on this dinoflagellate, a low grazing impact might be partially responsible for the long duration of these red tides. Therefore, whether there were effective predators on T. furca during its red tides of the year 2020 should be investigated.
The heterotrophic dinoflagellates Gyrodinium dominans, Oblea rotunda, Oxyrrhis marina, Pfiesteria piscicida, and naked ciliates are common heterotrophic protists in the waters of many countries, including Korea (Wood 1954, Hansen 1991, Jakobsen and Tang 2002, Mason et al. 2007, Lowe et al. 2010, Lim et al. 2017, Ok et al. 2017). Nevertheless, the worldwide distribution of these heterotrophic protists and whether they ingest T. furca has not been explored.
In the present study, we explored the interactions between T. furca and nine heterotrophic dinoflagellates (Aduncodinium glandula, Gyrodiniellum shiwhaense, Gyrodinium dominans, Gyrodinium jinhaense, Gyrodinium moestrupii, Luciella masanensis, Oblea rotunda, Oxyrrhis marina, and Pfiesteria piscicida), and one naked ciliate Rimostrombidium sp. These heterotrophic protists cover a wide range of size and feeding mechanisms (Hansen 1992, Strom and Buskey 1993, Sonntag et al. 2006, Jeong et al. 2007, 2011, Roberts et al. 2011, Jang et al. 2016, Kang et al. 2020). Furthermore, we investigated the abundance of T. furca and common heterotrophic protists in coastal-offshore waters off Yeosu in the South Sea of Korea on Jul 31, 2020, when huge T. furca red tides prevailed in the South Sea of Korea. The results of the present study will provide a better understanding of interactions between T. furca and heterotrophic protists and their population dynamics.
MATERIALS AND METHODS
Preparation of experimental organisms
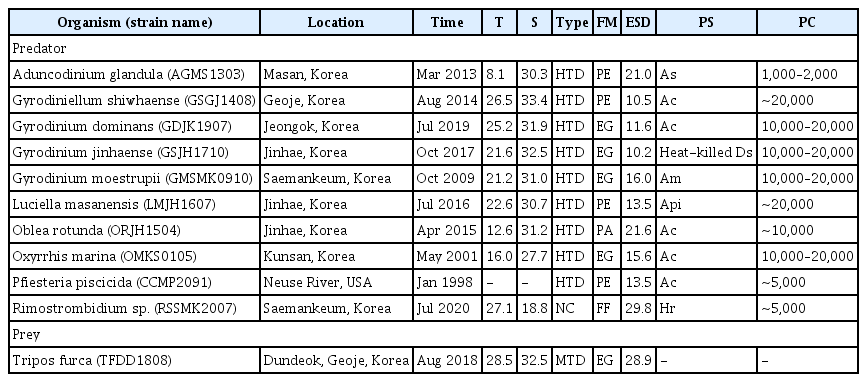
Tripos furca was isolated from surface waters off the coast of Dundeok, Geoje, southern Korea (34°51′26″ N, 128°28′34″ E) using plankton samplers in August 2018, when the water temperature and salinity were 28.5°C and 32.5, respectively (Table 1). The collected samples were gently screened using a 100 μm sieve and 10 μm Nitex mesh. By conducting two consecutive single-cell isolations, a T. furca TFDD1808 clonal culture was established. When T. furca concentration had increased sufficiently, it was transferred into 32, 270, and 500-mL polycarbonate (PC) bottles filled with fresh half-diluted L1 medium. The bottles were placed at 20°C, under a 14 : 10 h light : dark cycle, on a shelf illuminated with cool white fluorescent lights of 20 μmol photons m−2 s−1. Only cultures reaching the exponential growth stage were used in the study.
Information on the isolation and maintenance of the heterotrophic protists and Tripos furca used in the present study
The heterotrophic dinoflagellates A. glandula, G. shiwhaense, G. dominans, G. jinhaense, G. moestrupii, L. masanensis, O. rotunda, O. marina, and P. piscicida, isolated from plankton samples collected from coastal waters off Geoje, Jeongok, Jinhae, Kunsan, Masan, Neuse River, and Saemankeum from 1998 to 2019, were used in this study (Table 1). Two consecutive single-cell isolations were conducted to establish each heterotrophic dinoflagellate species clonal culture, except for that of P. piscicida CCMP2091, which was acquired from the National Center for Marine Algae and Microbiota, USA. The naked ciliate Rimostrombidium sp. was isolated from plankton samples collected in a bucket from the coastal waters off Saemankeum, Korea, in July 2020, when the water temperature and salinity were 27.1°C and 18.8, respectively (Table 1). A clonal culture for Rimostrombidium sp. was also set up with two serial single-cell isolations, and its volume increased from 6-well plate chambers to 50 and 270-mL PC bottles, with a diet of Heterocapsa rotundata as prey (ca. 5,000 cells mL−1 d−1).
The carbon content of T. furca TFDD1808 (1.7 ng C per cell) was estimated from the cell volume, according to the equation proposed by Menden-Deuer and Lessard (2000). Using the same calculation, the carbon contents of all predator species in the present study were also evaluated from the cell volume. The cell volumes of the heterotrophic predators were assessed using the methods of Jang et al. (2016) for A. glandula; Ok et al. (2017) for G. shiwhaense; Kang et al. (2020) for G. dominans, G. jinhaense, and G. moestrupii; Jeong et al. (2007) for L. masanensis and P. piscicida; You et al. (2020) for O. rotunda and O. marina; and this study for Rimostrombidium sp. (Table 1).
Interactions between Tripos furca and heterotrophic protists
This experiment was designed to examine whether each heterotrophic dinoflagellate or ciliate fed on T. furca TFDD1808, after mixing T. furca with potential predator species (Table 2). In this experiment, predation or other interactions between T. furca and the target heterotrophic protist were observed.
Dense cultures of T. furca (ca. 2,000 cells mL−1) and the target heterotrophic protist were added to individual 42-mL PC bottles (Table 2). One experiment bottle containing prey and predator, one prey control, and one predator control bottle were set up for the target heterotrophic protist. All the bottles, except for the ones with the A. glandula predator, were placed on a rotating wheel at 0.00017 g (0.9 rpm), while those for the A. glandula predator were placed on a shelf. All the bottles were incubated at 20°C, under a 14 : 10 h light : dark cycle, and illuminated with cool white fluorescent light of 20 μmol photons m−2 s−1.
Aliquots of 3 mL were taken from each bottle after 2, 24, and 48 h of incubation period, and placed into separate wells of 6-well plate chambers. More than 30 cells of each predator species were transferred into plate chambers and tracked for a minimum of 2 min under a dissecting microscope at 20–63× magnification to test whether the target predator fed on T. furca or not. The predator cells with and without ingested T. furca cells in their protoplasms were photographed on confocal dishes with cover glasses at 200–630× magnification using a digital camera (Zeiss-AxioCam 506; Carl Zeiss Ltd., Göttingen, Germany) attached to an inverted light microscope (Zeiss-Axiovert 200 M; Carl Zeiss Ltd.). The feeding process of the heterotrophic protists that successfully ingested T. furca cells was recorded and captured using an identical digital camera.
Abundance of Tripos furca and common heterotrophic protists in the waters off Yeosu
The two stations (Stations 501 and 504) were located in the nearshore and offshore regions off Yeosu in the South Sea of Korea (Fig. 1). The water depth for both sampling stations was approximately 20 m. Station 501 was located approximately 20 km south of the Seumjin River; thus, the salinity at this station sometimes reduced due to freshwater input (Jeong et al. 2017). Water temperature, salinity, dissolved oxygen (DO), and pH for each sampling depth were measured using a YSI Professional Plus instrument (YSI Inc., Yellow Springs, OH, USA).
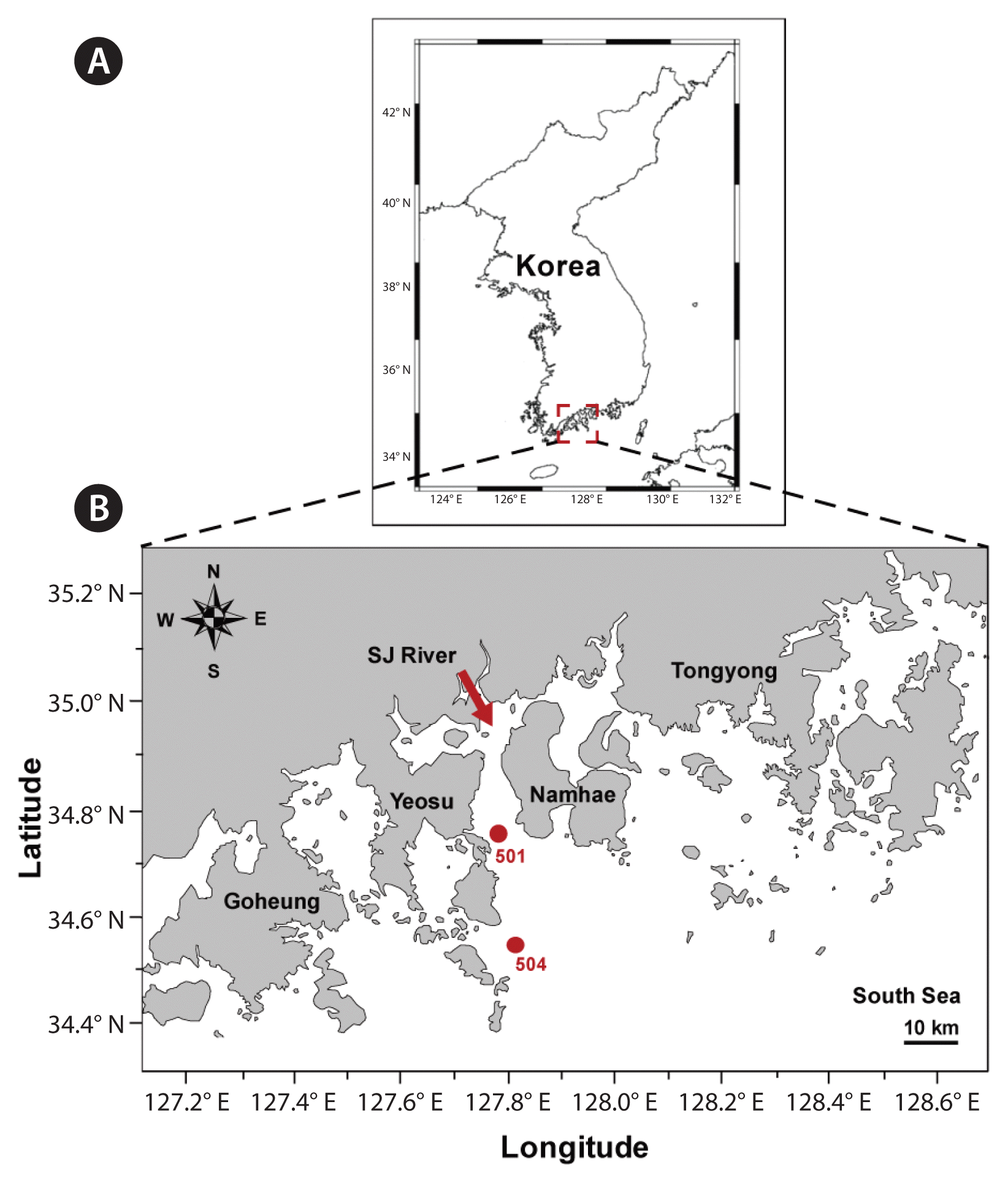
Maps of the study region and the sampling stations (A & B). The arrow between Yeosu and Namhae indicates Seomjin (SJ) River.
Water samples at Stations 501 and 504 were collected using water samplers at depths of 0, 5, 10, 15, and 20 m. The sampling time was 13:00 at Station 501 and 13:40 at Station 504. Plankton samples for counting were collected into 500-mL polyethylene bottles and preserved with acidic Lugol’s solution. To clarify the abundance of T. furca and heterotrophic protists, the fixed samples were concentrated by 1/5–1/10, using the settling and siphoning method (Welch 1948). After thorough mixing, all or a minimum of 100 cells of T. furca and each heterotrophic protist species in one to ten 1-mL Sedgwick-Rafter chambers were enumerated under a light microscope.
RESULTS
Interactions between Tripos furca and heterotrophic protists
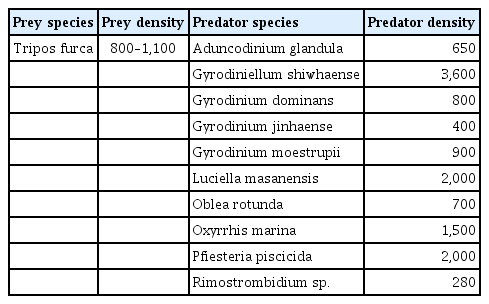
The heterotrophic dinoflagellates A. glandula, L. masanensis, and P. piscicida were able to feed on T. furca. However, the heterotrophic dinoflagellates G. shiwhaense, G. dominans, G. jinhaense, G. moestrupii, O. rotunda, O. marina, and the naked ciliate Rimostrombidium sp. were unable to feed on the prey (Table 3, Figs 2 & 3). Cells of A. glandula, L. masanensis, and P. piscicida successfully ingested T. furca using peduncles (Fig. 2). Multiple cells of L. masanensis or P. piscicida attacked a T. furca cell together (Fig. 2). However, G. jinhaense, G. moestrupii, O. rotunda, and O. marina attempted to capture T. furca, but failed to do so (Table 3, Fig. 3). Moreover, G. shiwhaense, G. dominans, and Rimostrombidium sp. did not attack T. furca (Table 3, Fig. 3). Regardless of capture by potential predators, T. furca did not support positive growth of any of the heterotrophic protists tested in the present study; thus, T. furca may be an undesirable food source for the common heterotrophic protists (Table 3).
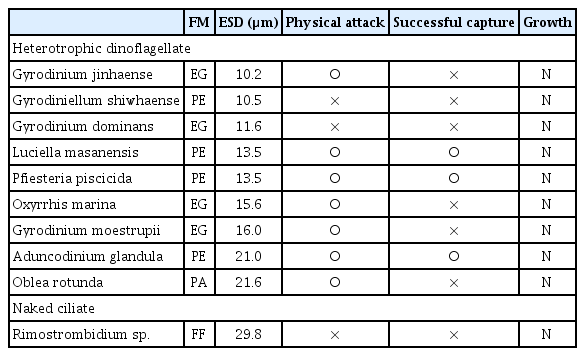
Taxa, size, and response of potential heterotrophic dinoflagellate and naked ciliate predators offered to Tripos furca (TFDD1808)
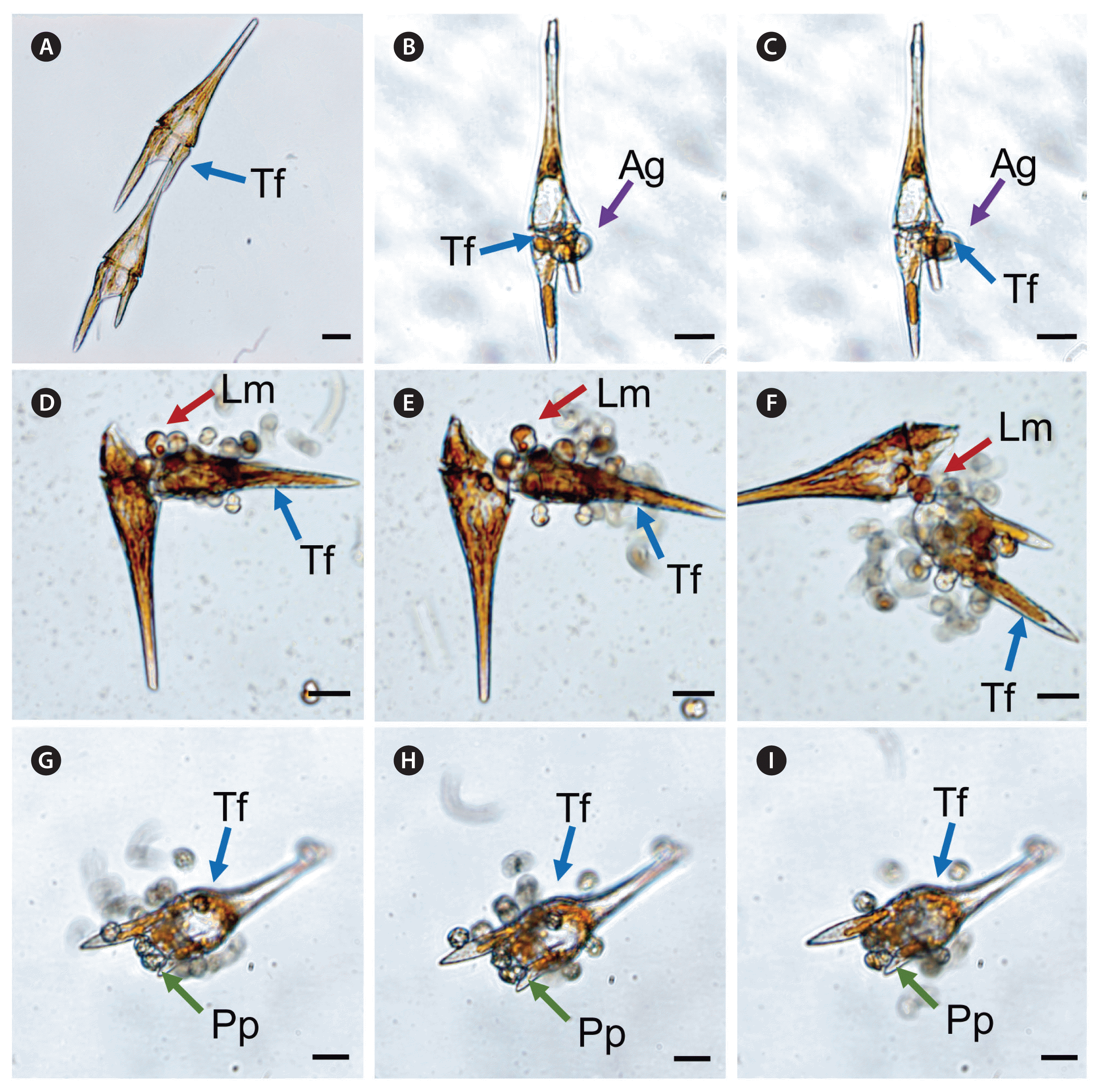
Successful feeding process of heterotrophic protists on Tripos furca (Tf ) recorded using a digital camera attached to an inverted light microscope. (A) Two Tf cells as prey control. (B & C) Feeding by Aduncodinium glandula (Ag) on a Tf cell. An Ag cell fed on a Tf cell using peduncle. (D–F) Feeding by Luciella masanensis (Lm) on a Tf cell. Multiple Lm cells fed on a Tf cell using peduncles. (G–I) Feeding by Pfiesteria piscicida (Pp) on a Tf cell. Multiple Pp cells fed on a Tf cell using peduncles. Scale bars represent: A–I, 20 μm.
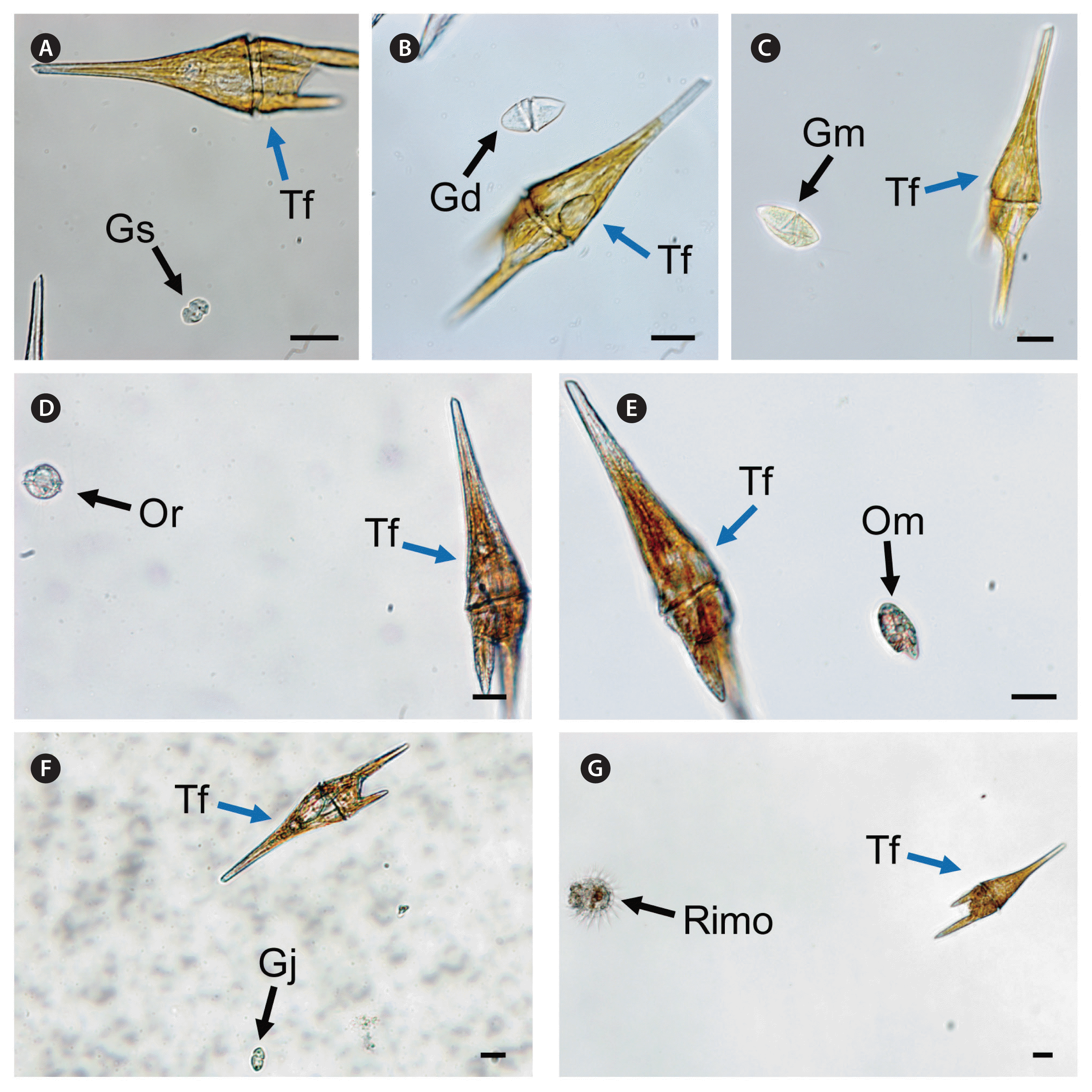
Unsuccessful feeding process of heterotrophic dinoflagellates on Tripos furca (Tf ) recorded using a digital camera attached to an inverted light microscope. (A) Gyrodiniellum shiwhaense (Gs) did not feed on Tf. (B) Gyrodinium dominans (Gd) did not feed on Tf. (C) Gyrodinium moestrupii (Gm) did not feed on Tf. (D) Oblea rotunda (Or) did not feed on Tf. (E) Oxyrrhis marina (Om) did not feed on Tf. (F) Gyrodinium jinhaense (Gj) did not feed on Tf. (G) Rimostrombidium sp. (Rimo) did not feed on Tf. Scale bars represent: A–G, 20 μm.
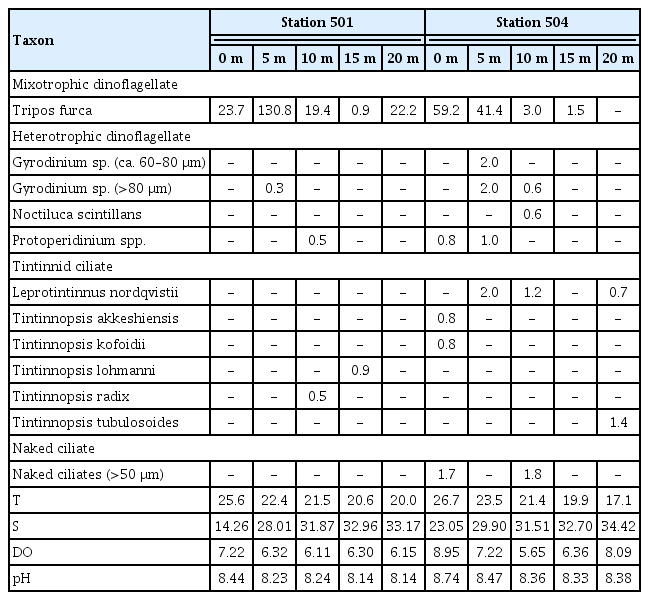
Abundance of Tripos furca and common heterotrophic protists in the waters off Yeosu on Jul 31, 2020
Tripos furca caused huge red tides in the South Sea of Korea from Jun 30 to Sep 5, 2020 (NFRDI data; http://www.nifs.go.kr/portal/redtideInfo). The water temperature and salinity at Station 501 on Jul 31, 2020, ranged from 20.0 to 25.6°C and 14.26 to 33.17, respectively, while those at Station 504 ranged from 17.1 to 26.7°C and 23.05 to 34.42, respectively (Table 4). The DO and pH at Station 501 on Jul 31, 2020, ranged from 6.11 to 7.22 mg L−1 and 8.14 to 8.44, respectively, while those at Station 504 ranged from 5.65 to 8.95 mg L−1 and 8.33 to 8.74, respectively (Table 4). The abundance of T. furca at Station 501 was 0.9 to 130.8 cells mL−1, but that at Station 504 was 0 to 59.2 cells mL−1 (Table 4). The abundance of the heterotrophic dinoflagellates Gyrodinium sp. (approximately 60–80 μm in cell length) and Gyrodinium sp. (>80 μm in cell length), both 2.0 cells mL−1, were observed at 5 m of Station 504, where an abundance of the heterotrophic dinoflagellates Protoperidinium spp., 1.0 cells mL−1, was found (Table 4). The maximum abundance of the heterotrophic dinoflagellates was 5.0 cells mL−1. The highest abundance of total tintinnids, 2.1 cells mL−1, was found at 20 m at Station 504, and Leprotintinnus nordqvistii was the most dominant tintinnid (Table 4). Naked ciliates >50 μm were found within 10 m at Station 504, with a maximum abundance of 1.8 cells mL−1 (Table 4).
DISCUSSION
Prior to the present study, the mixotrophic dinoflagellate Fragilidium subglobosum and heterotrophic dinoflagellates Noctiluca scintillans, Polykrikos kofoidii, and Protoperidinium steinii were reported to feed on T. furca (Hansen and Nielsen 1997, Jeong et al. 2001, Olseng et al. 2002, Drits et al. 2013). Fragilidium spp. are known to engulf whole prey through the sulcal area, N. scintillans engulf prey cell using mucus attached to the tentacle, and Polykrikos spp. engulf prey cells after anchoring the prey with a nematocyst-taeniocyst complex (Kiφrboe and Titelman 1998, Hansen and Calado 1999, Tillmann and Hoppenrath 2013). However, Protoperidinium spp. feed on prey cells wrapped in pallium after anchoring prey using a tow filament (Jacobson and Anderson 1992, Hansen and Calado 1999). Interestingly, the results of the present study clearly showed that among the common heterotrophic protist predators tested, only peduncle-feeders Aduncodinium glandula, Luciella masanensis, and Pfiesteria piscicida ingested T. furca TFDD1808. In contrast, the engulfment feeders Gyrodinium dominans, G. jinhaense, G. moestrupii, and Oxyrrhis marina, the filter feeder Rimostrombidium sp., and the pallium feeder Oblea rotunda did not consume T. furca. However, not all peduncle feeders were successful at feeding on T. furca; one of the peduncle feeders, Gyrodiniellum shiwhaense, failed to ingest T. furca. Therefore, feeding mechanisms may not be primarily responsible for the ability and inclination of these common heterotrophic protists to feed on T. furca. Cells of A. glandula are known to feed on diverse prey items, including perch blood cells and many algal prey species (Jang et al. 2016). The results of the present study extend the prey species of A. glandula to T. furca. Cells of L. masanensis and P. piscicida are known to feed on dinoflagellate prey species of ≤9.7 μm in the equivalent spherical diameter (ESD) and athecate dinoflagellates such as Margalefidinium polykrikoides, Akashiwo sanguinea, and Gymnodinium catenatum, but they did not feed on thecate dinoflagellates ≥12.1 μm (Jeong et al. 2007). You et al. (2020) reported that P. piscicida fed on larger sized thecate dinoflagellates, but only on motionless cells. Thus, the present study is the first to report that L. masanensis and P. piscicida are able to feed on a moving thecate dinoflagellate ≥12.1 μm because the ESD of T. furca is approximately 29 μm (Jeong et al. 2001). Cells of L. masanensis and P. piscicida swarmed to feed on a T. furca cell. Other peduncle feeding heterotrophic dinoflagellates Stoeckeria algicida and Stoeckeria changwonensis are known to swarm to feed on a prey cell (Jeong et al. 2005, Lim et al. 2014). Their distinctive swarming behaviors may be triggered by the chemosensory signals from prey (Burkholder and Glasgow 1995, Lewitus et al. 1999).
Among the heterotrophic protists that did not predate T. furca in the present study, G. shiwhaense, G. dominans, and Rimostrombidium sp. did not attempt to attack T. furca, whereas the other heterotrophic protists attempted to feed on T. furca cells. Thus, G. shiwhaense, G. dominans, and Rimostrombidium sp. may not have enzymes related to the detection of T. furca cells. Cells of G. jinhaense are known not to feed on microalgae >26 μm in ESD, except the dinoflagellate Lingulodinium polyedra, where ESD is approximately 38 μm (Kang et al. 2020). Thus, G. jinhaense may have difficulty engulfing T. furca cells. Furthermore, G. moestrupii cells were also observed to have difficulty in utilizing long T. furca cells as prey, although G. moestrupii is known to feed on L. polyedra (Kang et al. 2020). Cells of O. rotunda and O. marina successfully target dinoflagellate prey species <27 μm in ESD, respectively (Strom and Buskey 1993, Kang et al. 2019, Kim et al. 2019, You et al. 2020). Therefore, the elongated horns, and large size of T. furca may be partially responsible for non-feeding by these heterotrophic dinoflagellates.
Aduncodinium glandula, L. masanensis, and P. piscicida were not found in the water samples collected from two stations on Jul 31, 2020. In the present study, T. furca did not support the positive growth of these three heterotrophic dinoflagellates. Therefore, the results of the feeding experiments in the present study may provide an explanation for the non-detection of these three heterotrophic dinoflagellates in the water samples. The heterotrophic dinoflagellates Gyrodinium sp. (>80 μm) and Protoperidinium spp. and two tintinnid ciliates were found in the water samples collected from Station 501, at which the maximum abundance of T. furca was 130.8 cells mL−1. Meanwhile, Gyrodinium spp. (≥60 μm), Noctiluca scintillans, Protoperidinium spp., and several tintinnid species were found in the water samples collected from Station 504, at which the maximum abundance of T. furca was 59.2 cells mL−1. Furthermore, the abundances of Gyrodinium spp. (≥60 μm), N. scintillans, Protoperidinium spp., and several tintinnid species at Station 504 were as low as 0.6–2.0 cells mL−1. In contrast, Polykrikos kofoidii was not found in these water samples. Jeong et al. (2001) reported that P. kofoidii grew on T. furca with a maximum growth rate of 0.35 d−1, and half-saturation constant (the prey concentration sustaining 1/2 maximum growth rate) and the threshold prey concentration of P. kofoidii feeding on T. furca was 128 ng C mL−1 (102 cells mL−1) and 113 ng C mL−1 (90 cells mL−1), respectively. When the abundance of T. furca was 59.2 and 130.8 cells mL−1, the growth rates of P. kofoidii feeding on T. furca, calculated using the equation of Jeong et al. (2001), were −0.153 and 0.101 d−1, respectively. Therefore, low abundances of T. furca during the study period may be partially responsible for the lack of detection of Polykrikos spp.
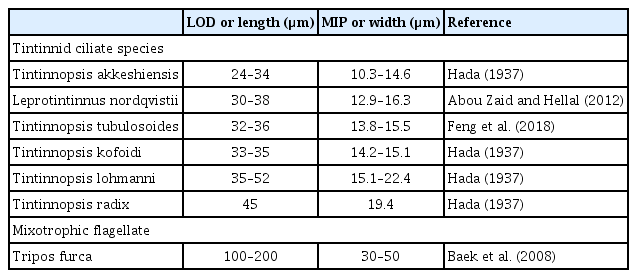
The naked ciliate Rimostrombidium sp. (oral diameter = 29.4 μm, n = 6) did not consume T. furca. The large tintinnid ciliates Favella philippinensis and Favella ehrenbergii (lorical oral diameter [LOD] = 69–144 μm) are known to engulf Tripos sp., while the small tintinnid ciliate Tintinnopsis uruguayensis (LOD = 37–42 μm) is unable to feed on T. furca (Godhantaraman and Krishnamurthy 1997, Sivasankar et al. 2017). The LOD of tintinnids is known to strongly correlate with the maximum and optimal prey size (Dolan 2010). In general, tintinnids are known to ingest maximum prey size up to roughly 43% of their LOD (Heinbokel 1978). To ingest whole T. furca cells, the LOD of tintinnids should be wider than approximately 70 μm. However, the tintinnids found in the sample waters (LOD = 24–52 μm) may not be large enough to feed on T. furca (Table 5). Thus, in the collected waters, the tintinnid ciliates may not be large enough to engulf T. furca. Therefore, T. furca was unlikely to support the rapid growth of the tintinnids at Stations 501 and 504 on Jul 31, 2020.
Conclusively, among the nine common heterotrophic dinoflagellates and one naked ciliate tested in the present study, only A. glandula, L. masanensis, and P. piscicida were found to feed on T. furca. However, T. furca did not support the positive growth of any of these predator species. Furthermore, the abundances of the heterotrophic protists that were able to feed on T. furca were low at Stations 501 and 504 on Jul 31, 2020. Therefore, predators may not significantly reduce the abundance of T. furca, which may be partially responsible for the prevalence of T. furca on the coast of the South Sea from June to September 2020.
ACKNOWLEDGEMENTS
We thank Ju Young Park, Dong Gyu Lee, and Eun Chong Park for technical support. This research was supported by the Useful Dinoflagellate program of Korea Institute of Marine Science and Technology Promotion funded by the Ministry of Oceans and Fisheries and the National Research Foundation funded by the Ministry of Science and ICT (NRF-2017R1E1A1A01074419; NRF-2020M3F6A1110582) award to HJJ.
Notes
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest.