Effect of different concentrations and ratios of ammonium, nitrate, and phosphate on growth of the blue-green alga (cyanobacterium) Microcystis aeruginosa isolated from the Nakdong River, Korea
Article information
Abstract
Microcystis aeruginosa causes harmful algal blooms in the Nakdong River of Korea. We studied the effect of different concentrations and ratios of ammonium (NH4+), nitrate (NO3−), and phosphate (PO43−) on growth of this species in BG-11 medium: each nutrient alone, NO3− : NH4+ ratio, the N : P ratio with fixed total N (TN), and the N : P ratio with fixed total P (TP). The single nutrient experiments indicated that M. aeruginosa had the highest growth rate at NH4+ and NO3− concentrations of 500 μM, and at a PO43− concentration of 5 μM. The NO3− : NH4+ ratio experiments showed that M. aeruginosa had the highest growth rate at a ratio of 1 : 1 when TN was 100 μM and 250 μM, and the lowest growth rate at a ratio of 1 : 1 when the TN was 500 μM. The N : P ratio with fixed TN experiments indicated that M. aeruginosa had the highest growth rates at 50 : 1, 20 : 1, and 100 : 1 ratios when the TN was 100, 250, and 500 μM, respectively. In contrast, the N : P ratio with fixed TP experiments showed that M. aeruginosa had the highest growth rates at 200 : 1 ratio at all tested TP concentrations. In conclusion, our results imply that the NO3− : NH4+ ratio and the PO43− concentration affect the early stage of growth of M. aeruginosa. In particular, our results suggest that the maximum growth of M. aeruginosa is not simply affected by the NO3− : NH4+ ratio and the N : P ratio, but is determined by the TN concentration if a certain minimum PO43− concentration is present.
INTRODUCTION
The Nakdong River is the longest river in the Republic of Korea, and it supplies drinking water for 13 million people. In recent years, summer blooms of Microcystis aeruginosa in this river have occurred more frequently and had longer durations. The Korean government has designated M. aeruginosa as a hazardous cyanobacterium that must be controlled because it produces the toxin, microcystin as well as the compounds with unpleasant taste and odor, and because its blooms have caused fish and livestock mortality (Lee et al. 2013, National Institute of Environmental Research 2013, Ahn et al. 2015).
A high P concentration is considered the main cause of Microcystis blooms (Kim and Kang 1993, Lee et al. 1998). Schindler et al. (2008) and Schindler (2012) emphasized that N is unlikely to be the limiting factor for blooms because of the presence of N2-fixing cyanobacterium in water bodies. Moreover, when phosphate (PO43−) is released from the sediment during summer, Microcystis absorbs and stores it in bottom layer (Jacobson and Halmann 1982, Jung and Cho 2003a, 2003b), then moves toward the high-intensity light at the surface, using its gas vacuole, and thereby generates blooms (Reynolds et al. 1981, Conley et al. 2009, Ahn et al. 2015).
Other studies have focused on the importance on N in cyanobacterial blooms (Conley et al. 2009, Dolman et al. 2012, Paerl et al. 2014, Hammed et al. 2016). During summer, the ammonium (NH4+) concentration increases from the sediment (Jung and Cho 2003a, 2003b). Lee and Cho (2006) reported that NH4+ affects the size of Microcystis cells. Brookes and Ganf (2001) reported that Microcystis recovers its buoyancy more quickly when the nitrate (NO3−) concentration is higher. Several studies reported that a low NO3− : NH4+ ratio may promote Microcystis blooms (Liu et al. 2011, Dai et al. 2012). Thus, many studies have examined the effect of different concentrations and ratios of N and P on Microcystis proliferation and long-term growth (Park et al. 1993, Lee et al. 1998, Nalewajko and Murphy 2001, Vézie et al. 2002, Kim and Hwang 2004, Lee and Cho 2006, Baldia et al. 2007, Chen et al. 2009).
However, most these studies simply examined the effect of NO3− and PO43−, and did not consider NH4+ together (Lee et al. 1998, Brookes and Ganf 2001, Baldia et al. 2007). Furthermore, there are disagreements regarding the importance of the N : P ratio on cyanobacterial blooms (Scheffer et al. 1997, Xie et al. 2003, Kim and Hwang 2004) and about whether N or P has a more significant effect on growth of Microcystis (Conley et al. 2009, Schindler 2012, Kim et al. 2013). In particular, the P concentration in the Nakdong River has decreased significantly since 2012 due to the efforts of the Four Rivers Restoration Project to improve water quality. Nevertheless, Microcystis blooms have become more serious in recent years and have even begun to occur during winter. Therefore, the studies of other nutrients rather than P have been required (Yu et al. 2014, 2015).
In this study, we aimed to identify the effect of NO3−, NH4+, and PO43− on the growth of M. aeruginosa. We examined the effect of different concentrations of each nutrient alone, different NO3− : NH4+ ratios, and different N : P ratios to clarify the effects of N and P and the role of the N : P ratio on Microcystis growth. Finally, we analyzed our results in light of recent data from the Nakdong River to suggest a strategy that may help to control Microcystis blooms.
MATERIALS AND METHODS
Strain
We used a Microcystis aeruginosa strain that was collected from the Gangjeong-Goryeong weir in Dalseong-gun in Daegu, Republic of Korea on Oct 3, 2013 (Fig. 1). A colony was isolated using the capillary method (Guillard 1973). Identification was confirmed by morphological and molecular analysis, and the strain has been maintained at Kyungpook National University, Korea.
Culture conditions
M. aeruginosa cells were cultured in BG-11 medium (Stanier et al. 1971) (Table 1), but FeCl3·6H2O was substituted for ferric ammonium citrate. NaNO3, K2HPO4, and NH4Cl were used to regulate the concentrations of NO3−, PO43−, and NH4+, respectively, and other nutrients of BG-11 were controlled. Before each experiment, cells were adapted to a medium without N or P for a week. In each experiment, three 125-mL Erlenmeyer flasks with 100 mL of medium were autoclaved, and M. aeruginosa was inoculated at an initial cell density of 5,000 cells mL−1. All experiments were performed at a temperature of 30°C, light intensity of 67 ± 2 μmol photons m−2 s−1 on 16 : 8 h light-dark cycle, and at pH 8.0. The effects of NH4+, NO3−, and PO43− were tested in four sets of experiments: (1) different concentrations of each nutrient alone; (2) different NO3− : NH4+ ratios; (3) different N : P ratios with fixed total N (TN) concentration and variable P concentration (“N : P ratio with fixed TN”); and (4) different N : P ratios with fixed total P (TP) concentration and variable N concentration (“N : P ratio with fixed TP”). Furthermore, the NO3− : NH4+ ratio experiments and the N : P ratio with fixed TN experiments were performed at three levels of TN (100, 250, and 500 μM), and the N : P ratio with fixed TP experiments were performed at three levels of TP (1, 5, and 10 μM). In the all experiments for PO43− concentrations and N : P ratios, the NO3− : NH4+ ratio was 10 : 1. Table 2 summarizes the experimental conditions.
Cell counting and calculation of growth rate
M. aeruginosa cells were counted every 3 days using a light microscope (Axio Imager A1, Zeiss, Jena, Germany) and a hemocytometer (Marienfeld-Superior, Lauda-Königshofen, Germany) at a magnification of 200×. Each experiment lasted 24 days, at which the cells were in the stationary phase or death phase. After cell counting, the number of cells per unit volume and the growth rate were calculated. The maximum growth rate (μ) was calculated as: μ = ln (N2/N1)/(t2 − t1), where N2 and N1 indicate the cell density per unit volume at times t2 and t1 during the exponential growth phase (Levasseur et al. 1993).
Statistical analysis
All statistical analyses were conducted using the PASW (SPSS) statistics 18 software (SPSS Inc., Chicago, IL, USA). The results were analyzed by one-way ANOVA, two-way ANOVA, and Duncan’s post-hoc analysis. The results of all tests were considered significant for a p-value below 0.05.
RESULTS
Effect of NO3− and NH4+ concentration
The results for single condition of NO3− and NH4+ are shown in Fig. 2. The maximum growth rate of M. aeruginosa occurred at 500 μM NO3− (μ = 0.268 d−1) and 500 μM NH4+ (μ = 0.294 d−1) (p < 0.01 for each). Although NO3− and NH4+ concentrations significantly affected the growth of M. aeruginosa (p < 0.01), but the different forms of N had similar effects on that of this species (p = 0.388). Moreover, the results showed that a minimum concentration of 100 μM NH4+ or NO3− was necessary to grow at least 1,000,000 cells mL−1, a criterion for algal blooms established by the Korean algal-bloom warning system (National Institute of Environmental Research 2013).

Growth of Microcystis aeruginosa at different NO3− concentrations (A) and NH4+ concentrations (B), and maximum growth rates under all conditions (C). The PO43− concentration was controlled as 230 μM in these experiments. Asterisks above graphs of (A) and (B) denote significant differences in cell density among treatments for the indicated day based on one-way ANOVA (*p < 0.05 and **p < 0.01). Different letters above bars of (C) denote differences in maximum growth rate based on Duncan’s post-hoc analysis after an ANOVA revealed difference among conditions (p < 0.01). Here and below, error bars denote standard deviations of triplicate samples.
Effect of PO43− concentration
The results for single condition of PO43− are shown in Fig. 3. The maximum growth rate of M. aeruginosa was at 5 μM PO43− (μ = 0.480 d−1), and growth rates at higher concentrations than 5 μM PO43− were also high but slightly lower (p < 0.01). In addition, our results showed that a minimum of 1 μM PO43− was necessary to grow at least 1,000,000 cells mL−1.
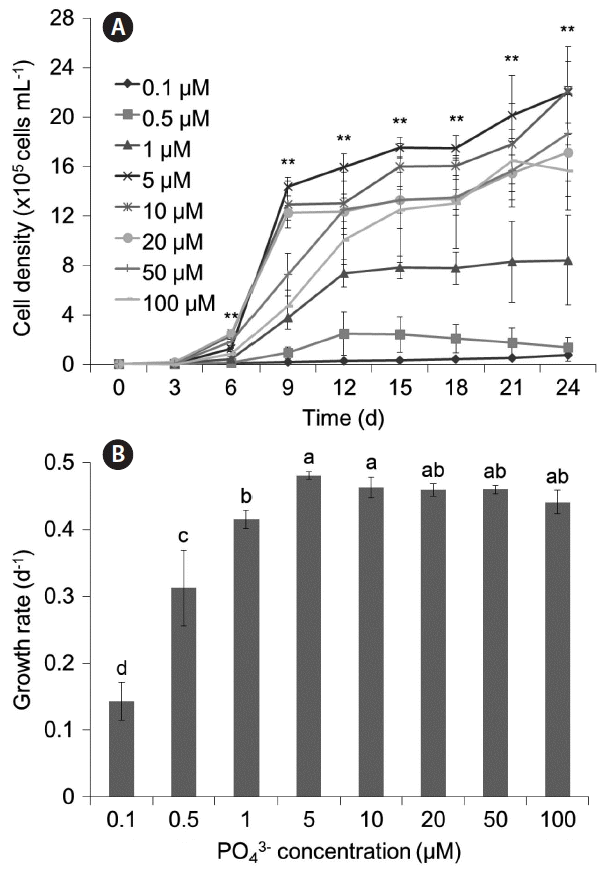
Growth of Microcystis aeruginosa at different PO43− concentrations (A) and maximum growth rates at different PO43− concentrations (B). The N concentration was controlled as 17.65 mM, and NO3− : NH4+ ratio was 10 : 1 in these experiments. Asterisks above graphs of (A) denote significant differences in cell density among treatments for the indicated day based on one-way ANOVA (**p < 0.01). Different letters above bars of (B) denote differences in maximum growth rate based on Duncan’s post-hoc analysis after an ANOVA revealed difference among conditions (p < 0.01).
Effect of NO3− : NH4+ ratio
The results for NO3− : NH4+ ratio of each TN level are shown in Fig. 4. At a TN concentration of 500 μM, the growth rate of M. aeruginosa was the lowest when the NO3− : NH4+ ratio was 1 : 1 (μ = 0.349 d−1) and the highest when this ratio was 100 : 1 (μ = 0.400 d−1). In contrast, the other experiments showed the highest growth rates for a NO3− : NH4+ ratio of 1 : 1 for a TN concentration of 100 μM (μ = 0.459 d−1), and 250 μM (μ = 0.421 d−1) (p < 0.01 for each comparison). Overall, the TN concentration had a significant effect on the growth of M. aeruginosa (p < 0.05), but the NO3− : NH4+ ratio had no such impact (p = 0.226). After 24 days, the cell density was not significantly different for the diverse ratios at each TN concentration (p = 0.411 for 100 μM TN; p = 0.880 for 250 μM TN; p = 0.204 for 500 μM TN). In addition, the cell density under each ratio became similar about 1,000,000 cells mL−1 when the TN was 100 μM, about 1,800,000 cells mL−1 when the TN was 250 μM, and about 2,500,000 cells mL−1 when the TN was 500 μM.

Growth of Microcystis aeruginosa at different NO3− : NH4+ ratios with a total N (TN) concentration of 100 μM (A), 250 μM (B), and 500 μM (C), and maximum growth rates under all conditions (D). The PO43− concentration was controlled as 230 μM in these experiments. Asterisks above graphs of (A), (B), and (C) denote significant differences in cell density among treatments for the indicated day based on one-way ANOVA (*p < 0.05 and **p < 0.01). Different letters above bars of (D) denote differences in maximum growth rate based on Duncan’s post-hoc analysis after an ANOVA revealed difference among conditions (p < 0.01).
Effect of N : P ratio with fixed TN
The results for N : P ratio of each TN level are shown in Fig. 5. The maximum growth rate was at an N : P ratio of 50 : 1 when the TN was 100 μM (μ = 0.357 d−1), at an N : P ratio of 20 : 1 when the TN was 250 μM (μ = 0.375 d−1), and at an N : P ratio of 100 : 1 when the TN was 500 μM (μ = 0.378 d−1) (p < 0.01 for each comparison). Overall, the TN concentration (p < 0.05) and the N : P ratio (p < 0.01) each had effects on the growth of M. aeruginosa. However, after 24 days, the cell density was not statistically different for the diverse N : P ratios (p = 0.133 for 100 μM TN; p = 0.255 for 250 μM TN; p = 0.143 for 500 μM TN). The cell density under each ratio also became similar about 800,000 cells mL−1 when the TN was 100 μM, about 1,500,000 cells mL−1 when the TN was 250 μM, and about 2,000,000 cells mL−1 when the TN was 500 μM.
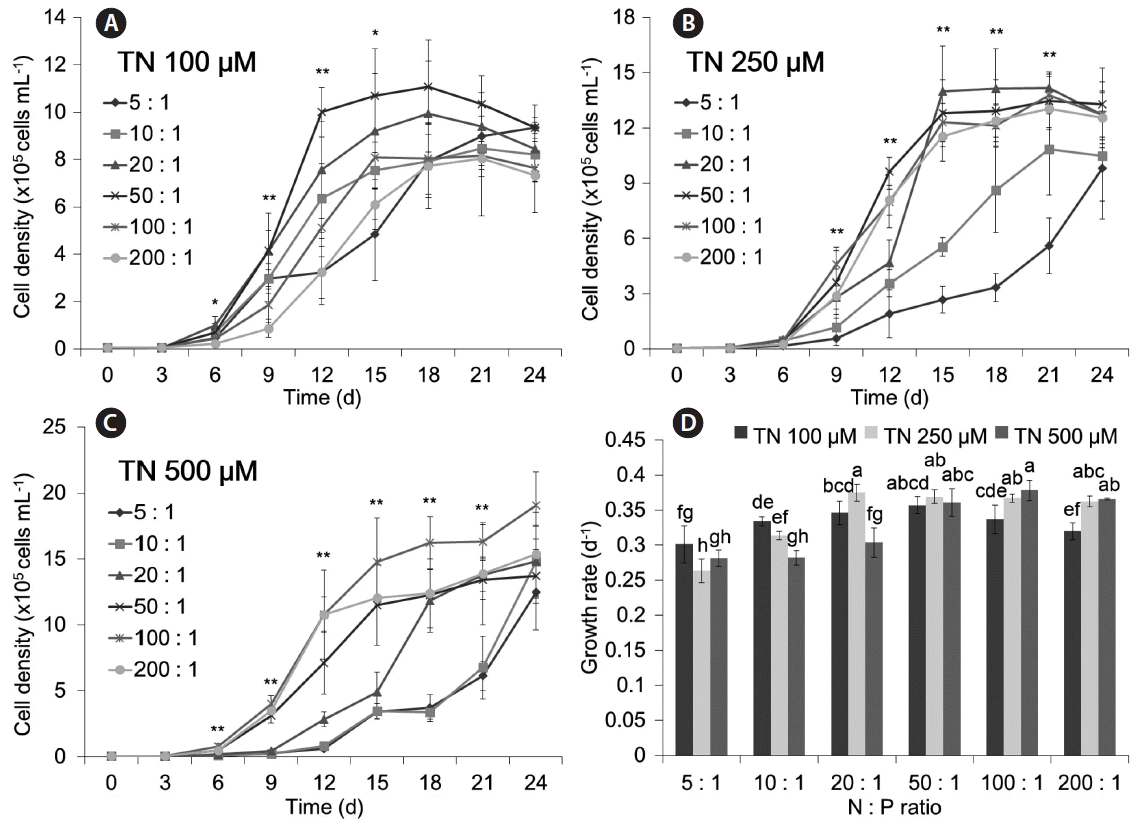
Growth of Microcystis aeruginosa at different N : P ratios with a fixed total N (TN) concentration of 100 μM (A), 250 μM (B), and 500 μM (C), and maximum growth rates under all conditions (D). The NO3− : NH4+ ratio was 10 : 1 in these experiments. Asterisks above graphs of (A), (B), and (C) denote significant differences in cell density among treatments for the indicated day based on one-way ANOVA (*p < 0.05 and **p < 0.01). Different letters above bars of (D) denote differences in maximum growth rate based on Duncan’s post-hoc analysis after an ANOVA revealed difference among conditions (p < 0.01).
Effect of N : P ratio with fixed TP
The results for N : P ratio of each TP level are shown in Fig. 6. At all 3 tested P concentrations, the highest population growth and growth rate were at an N : P ratio of 200 : 1 (μ = 0.433 d−1 for 1 μM TP; μ = 0.447 d−1 for 5 μM TP; μ = 0.475 d−1 for 10 μM TP) (p < 0.01 for each comparison). Thus, the TP concentration (p < 0.01) and the N : P ratio (p < 0.01) significantly affected the growth of M. aeruginosa.
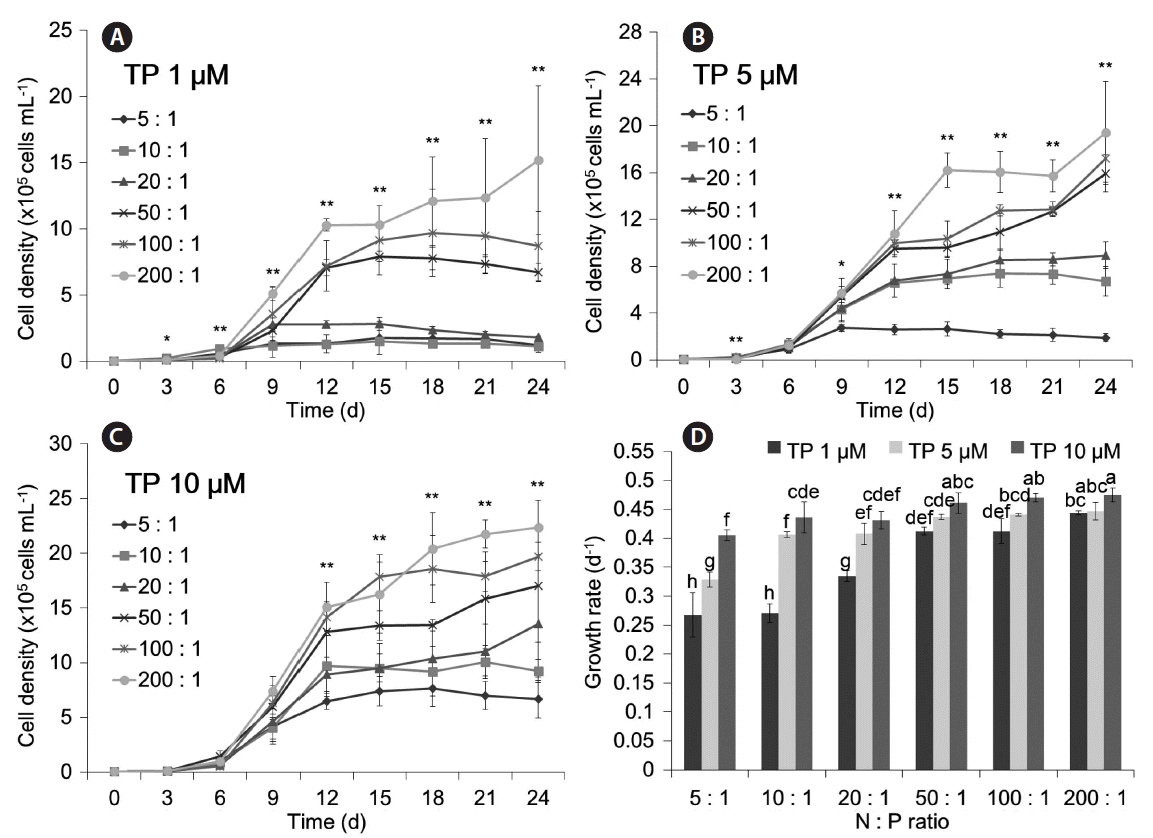
Growth of Microcystis aeruginosa at different N : P ratios with a fixed total P (TP) concentration of 1 μM (A), 5 μM (B), and 10 μM (C), and maximum growth rates under all conditions (D). The NO3− : NH4+ ratio was 10 : 1 in these experiments. Asterisks above graphs of (A), (B), and (C) denote significant differences in cell density among treatments for the indicated day based on one-way ANOVA (*p < 0.05 and **p < 0.01). Different letters above bars of (D) denote differences in maximum growth rate based on Duncan’s post-hoc analysis after an ANOVA revealed difference among conditions (p < 0.01).
DISCUSSION
Our experiments indicated that the growth of M. aeruginosa increased as the NO3− and NH4+ concentration increased, in agreement previous studies (Vézie et al. 2002, Lee and Cho 2006, Chen et al. 2009). Rücker and Giani (2004) reported that NH4+ had a greater effect than NO3− on the early growth of Microcystis, and that the growth rate was greater for NO3− than NH4+. Our results also showed that NH4+ promoted slightly faster cell growth initially, but the difference of NO3− and NH4+ did not differently affected the growth of M. aeruginosa.
The results of our experiments on the effect of the PO43− concentration are in agreement with previous studies (Park et al. 1993, Lee et al. 1998), which reported that a minimum of 0.05 mg L−1 (1.5 μM PO43−) is necessary for M. aeruginosa growth, and 0.3–0.8 mg L−1 (10–30 μM PO43−) is needed for a high growth rate. Baldia et al. (2007) reported that the growth rate of M. aeruginosa increased with N concentrations up to of 620 μM (8.7 mg L−1) and with P concentrations up to 7 μM (0.22 mg L−1). Our results also indicated that maximum growth of M. aeruginosa occurred at a relatively high N concentration, but at a relatively low P concentration.
When algae absorb NH4+, they immediately incorporate it into amino acids; however, algae can only use NO3− after enzymatic reduction to NO2− and NH4+, and these enzymatic reactions require cellular energy and thereby affect cell growth (Flynn et al. 1997, Flores et al. 2005). Therefore, algae that use NH4+ before NO3− (Takamura et al. 1987, Liu et al. 2011) may experience inhibition of NO3− uptake (Dortch 1990, Dugdale et al. 2007). Similarly, our results showed that M. aeruginosa had a lower growth rate at a TN concentration of 500 μM, indicating that decreased NO3− absorption in the presence of NH4+ seemed to hinder the growth of M. aeruginosa. However, Dortch (1990) reported that inhibition of NO3− uptake by NH4+ and the preference for NH4+ uptake vary according to environmental conditions and species. We observed a similar effect for a NH4+ concentration below 250 μM, suggesting that NH4+ might inhibit NO3− uptake at concentrations above 250 μM. However, this effect only occurred during the initial growth phase, and cell densities at 24 days were similar for different TN concentrations. Therefore, our results suggest that the TN concentration has a significant role in the growth of M. aeruginosa than the form of N.
We performed two sets of experiments to determine the effect of the N : P ratio on growth of M. aeruginosa. The first set of experiments used different N : P ratios with fixed TN, and indicated that the highest growth rate was at an N : P ratio of 20 : 1 when the TN was 250 μM, similar to the results of Kim and Hwang (2004) and Lee and Cho (2006), and the highest growth rate was at an N : P ratio of 100 : 1 when the TN was 500 μM, similar to the results of Nalewajko and Murphy (2001). However, the highest population growth rate was at an N : P ratio of 50 : 1 when the TN was 100 μM, in contrast to the results of previous studies (Nalewajko and Murphy 2001, Kim and Hwang 2004, Lee and Cho 2006). Although our results were different from these previous results, their P concentrations were in the range of 2 to 12.5 μM, and the results are similar to the results of our experiments in which PO43− alone was varied (Fig. 3). Therefore, it seems that the PO43− concentration affects the growth of M. aeruginosa rather than the N : P ratio. However, this effect was limited to the initial growth phase and each cell density became similar for different TN concentrations after 24 days.
Our second set of N : P ratio experiments used different N : P ratios with fixed TP, and showed that the highest growth rates occurred at the N : P ratio of 200 : 1 in all case. Kim et al. (2013) reported that there was no significant relationship between growth of M. aeruginosa and N : P ratio. Likewise, our results suggested that the N : P ratio itself did not determine the growth of M. aeruginosa in that different results were obtained in the two sets of experiments. In addition, some studies reported that differences in the growth of Microcystis at different N : P ratios are due to difference in the TP concentration (Scheffer et al. 1997, Kim and Hwang 2004). However, our results suggest that increasing the PO43− concentration above 1 μM had no clear effect on growth of M. aeruginosa, and only the TN concentration affected cell growth when a minimum PO43− concentration was present. In other words, it seems that the absolute amount of N and P, rather than the N : P ratio, affects the growth of M. aeruginosa, and the N concentration is more critical than the P concentration.
Unlike other cyanobacterium, Microcystis cannot fix atmospheric N2 and relies on N in the water. However, this species can store extra P within its cells (Reynolds et al. 1981, Xie et al. 2003, Kim and Hwang 2004). Moreover, P-limited conditions have a less effect on small size organisms, such as Microcystis, than larger organisms because of the advantage of diffusion through an aqueous boundary layer into cell (Chisholm 1992, Lin et al. 2016). Choi and Kim (2000) also reported that Microcystis can produce organophosphate-degrading enzymes, therefore, it can use other forms of P. As a consequence, the P concentration seems less important than the N concentration for promotion of the higher growth of Microcystis.
Most of the N and P in the Nakdong River are in the forms of NH4+, NO3−, and PO43−. Moreover, over the past 5 years, this river has had an average the total dissolved N about 215 μM (3.02 mg L−1), and an average total dissolved P of about 1.1 μM (0.035 mg L−1) (Water Information System, National Institute of Environmental Research, Korea 2016). Owing to the efforts of the Four Rivers Restoration Project, the P concentration has remained at about 0.4 μM (0.012 mg L−1) in winter, spring, and late fall. However, the P concentration has increased to about 1.5 μM (0.048 mg L−1) every summer and early fall, when most Microcystis blooms have occurred. Moreover, the amount of N, which has a greater effect on growth of Microcystis as shown in our results, has remained at 142 μM (1.98 mg L−1) or more in every season (Fig. 7A). Therefore, the concentrations of N and P in the Nakdong River are likely to be sufficient to support summer blooms of Microcystis. Furthermore, the Nakdong River has had trends of gradual decrease in the N : P ratio and the NO3− : NH4+ ratio from winter to summer of each year (Fig. 7B). However, as shown in our results, the change of N : P ratio in this river might not play a vital role in Microcystis blooms. Instead, the change of N : P ratio may be just a result from the that of the P concentration and the N concentration. In addition, the change of the NO3− : NH4+ ratio and the increased level of NH4+ in summer may favor the initial growth of Microcystis and contribute to explosive its blooms.
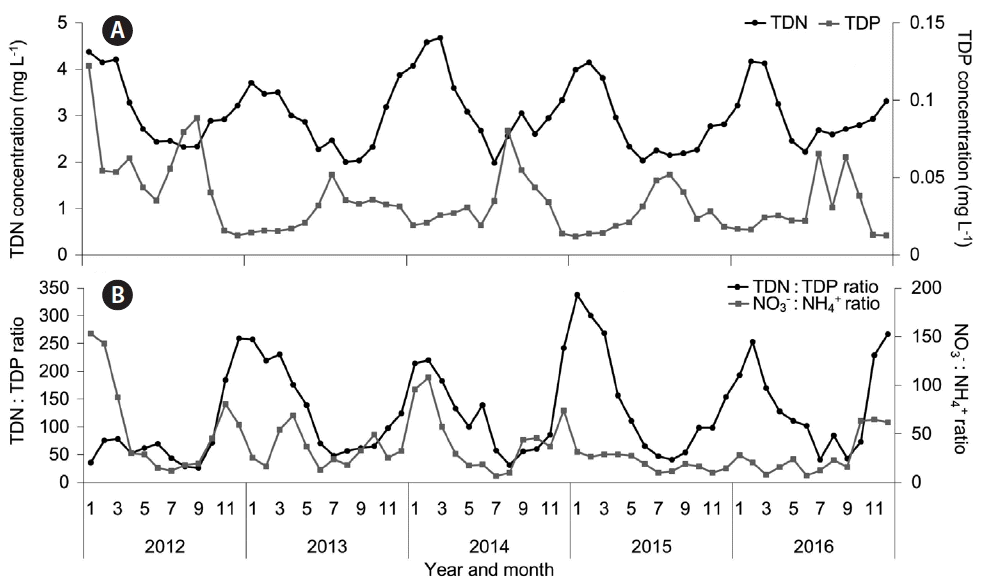
Average nutrient levels in 5 sites (Changnyeong-Haman Weir, Dalseong Weir, Dodongseowon, Gangjeong-Goryeong Weir, and Hapcheon-Changnyeong Weir) of the Nakdong River (Korea) over the past 5 years. Total dissolved nitrogen (TDN) and total dissolved phosphorus (TDP) (A), and the TDN : TDP ratio and the NO3− : NH4+ ratio (B). Data are from the Water information system (Water Information System, National Institute of Environmental Research, Korea 2016).
In conclusion, we suggest that the PO43− concentration in the Nakdong River should be reduced to below 1 μM during summer and early autumn to prevent the formation of Microcystis blooms. Alternatively, the N concentration should be regulated to reduce the growth of Microcystis while maintaining the P concentration at its current level. However, the physiology of Microcystis is incompletely understood, and the N and P cycles are complicated in Nakdong River than in controlled laboratory experiments. Therefore, further studies are required to figure out physiological characteristics of Microcystis, to identify exact cause of the change of NH4+, NO3− and PO43−, and to develop effective strategies for control of Microcystis blooms.
ACKNOWLEDGEMENTS
This research was supported by Kyungpook National University Bokhyeon Research Fund, 2015.


