FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae
Article information
Abstract
Polysaccharides of marine algae exhibit different structural characteristics and interesting biological functions. In this study, crude polysaccharides (CP) of eleven Sri Lankan marine algae obtained through hot water extraction and ethanol precipitation were investigated for DPPH, alkyl, and hydroxyl radical scavenging activities using electron spin resonance spectrometry and for intracellular reactive oxygen species scavenging activity in the Chang liver cell line. Characterization of CPs was done by Fourier transform infrared (FTIR) spectroscopy and by analysis of the monosaccharide composition. Time-dependent density functional theory quantum-chemical calculations at the RB3LYP/6-31G(d,p) level for constructed dimeric units of the corresponding polysaccharides were used to resolve the FTIR spectra. CPs from Chnoospora minima showed the highest DPPH and alkyl radical scavenging activities and higher intracellular reactive oxygen species scavenging effects for both AAPH and H2O2 induced ROS production in “Chang” cells. The major polysaccharide constituent in C. minima CP was identified as fucoidan and it displayed a higher sulfate content. The degree of sulfation of these polysaccharides suggests a positive correlation with the observed antioxidant properties.
INTRODUCTION
The ocean covers more than 70% of Earth’s surface and is characterized by a wide diversity of marine organisms that offer a rich source of natural products (Wijesekara et al. 2011). Many wonders of this unique environment still remain a mystery. According to recent findings, marine organisms are rich in bioactive compounds that include polysaccharides, polyunsaturated fatty acids, polyphenolic compounds, antioxidants, peptides, essential vitamins and minerals (Heo et al. 2006, Kim et al. 2014, Lee et al. 2015). Sulfated polysaccharides (SPs) purified from algae and other organisms in particular, have been widely used in food, cosmetic, and pharmaceutical industries due to their broad spectrum of bioactivity and limited toxicity (Fleita et al. 2015). These macromolecules possess anticoagulant, antiviral, antioxidant, anticancer and immunomodulatory activities (Wijesekara et al. 2011, Nishiguchi et al. 2014, Kandasamy et al. 2015). They are mainly located in the cell walls of algae. Major SPs include fucoidans, laminaran and alginates from brown algae, carrageenans and agar from red algae and galactans, mannans and xylans from green algae (Percival 1979). The anti-oxidant activities of these bio-polymers have become an interesting research topic due to the role played by these molecules in defending the body against reactive oxygen species (ROS) (Vijayabaskar et al. 2012). Major ROS in biological systems include the superoxide radical, hydrogen peroxide, and the hydroxyl radical. They are generated during normal cellular metabolic processes and during pathogenic attacks (Yu 1994). Although enzyme mediated antioxidant cellular defense mechanisms exist, excessive production of ROS causes oxidative stress and cell damage.
Crude polysaccharides (CPs) and their enzyme hydrolysates from marine algae have shown interesting antioxidant properties. CPs composed of sulfated uronic acid residues from the brown alga Turbinaria ornata have demonstrated profound 2,2-diphenyl-1-picrylhydrazy (DPPH), nitric oxide and ABTS+ radical scavenging activity and lipid peroxidation inhibition activities (Ananthi et al. 2010). Fucoidan, an SP extracted from Ecklonia cava has shown in vitro and in vivo anti-inflammatory activity in lipopolysaccharide induced RAW 264.7 macrophages and in zebrafish (Lee et al. 2013). SP from Sargassum swartzii reports being a good source of natural antioxidants with promising DPPH, ABTS+, and H2O2 radical scavenging activities (Vijayabaskar et al. 2012). Unraveling the structural, compositional and sequential properties of these bioactive polysaccharides has become one of the major focuses of recent biochemical research (Pereira et al. 2013).
Sri Lankan marine algae have not been widely explored except in a few biochemical and ecological studies. Relevant literature includes the ecological and taxonomical study by Durairatnam (1961) and a study on the distribution and morphological features of Sri Lankan macroalgae (Coppejans et al. 2009). The earliest biochemical analysis focused on the identification of sterols from 18 Sri Lankan algae samples (Mahendran et al. 1980). Recently, attention has been payed to the biochemical properties and natural products of these algae. Lakmal et al. (2014) have reported the anticancer and antioxidant effects of several Sri Lankan marine algae including Chondrophycus ceylanicus, Gelidiella acerosa, Gracilaria corticata, Chaetomorpha crassa, Caulerpa racemose, and Sargassum cassifolium. Premakumara et al. (1996) have studied the post-coital contraceptive activity of crude extracts of G. corticata and G. acerosa and have isolated a non-steroidal contragestative agent, a sphingosine derivative from Gelidiella acerosa. There are no previous reports on the properties of polysaccharides from Sri Lankan algae. The aim of this study was to investigate the CPs of overlooked marine algae of the Sri Lankan coastal waters and explore their antioxidant properties.
MATERIALS AND METHODS
Materials
Polysaccharide standards (alginic acid from brown algae, A7003; fucoidan from Fucus vesiculosus, F5631; ι-carrageenan, C1138; agar, 56763) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All organic solvents used during the sample preparation were of analytical grade. Chang liver cells were purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) was purchased from Gibco Inc. (Grand Island, NY, USA). Potassium bromide (Fourier transform infrared [FTIR] grade) was purchased from Sigma-Aldrich.
Collection of algal samples
Chaetomorpha antennina (SLGS1), Halimeda discoidea (SLGS2), Halimeda gracilis (SLGS3), Gracilaria corticata var. ramalinoides (SLRS5), Gracilaria foliifera (SLRS6), Ahnfeltiopsis pygmaea (SLRS7), Gracilaria corticata (SLRS8), and Jania adhaerens (SLRS9) were collected from the cost of Galle, Sri Lanka (6°4′54.19″ N/80°8′51.78″ E). Caulerpa racemosa var. racemosa f. remota (SLGS4) was collected from the cost of Hikkaduwa, Sri Lanka (6°4′54.19″ N/80°8′51.78″ E) and Chnoospora minima (SLBS11), and Gracilaria edulis (SLRS10) were collected from the coast of Kalpitiya, Sri Lanka (6°4′54.19″ N/80°8′51.78″ E). Samples were identified by Dr. Chandrika Nanayakkara, a specialist in algal identification based on morphological and anatomical characters. Repositories were stored in the herbarium at the University of Colombo. Samples were washed thoroughly to remove any attached epiphytes and debris. Subsequently, samples were lyophilized, ground into a fine powder, and stored at −20°C until further use.
Extraction of crude polysaccharides (CPs)
Algae powders (5.0 g each) were depigmented with acetone and extracted twice using distilled water at 90–95°C under continuous shaking for 3–4 h. Extracts were vacuum filtered and concentrated to one-fourth of the original volume. CPs were precipitated from extracts by adding three volumes of 95% ethanol bringing it up to the original volume. Mixtures were allowed to stand for 8 h at 4°C. Precipitated CPs were separated by centrifugation (12,000 rpm) at 4°C. Hereafter the precipitate will be referred to as CP fraction.
Chemical analysis
The proximate composition of the 11 algal samples was analyzed according to the Association of Official Analytical Chemists (AOAC) 2005 methods. Accordingly, the protein content was determined with the standard Kjeldahl method, the lipid content with the Soxhlet method, and the ash content by dry ashing in a furnace at 550°C for 6 h (Horwitz and Latimer 2005). The total polysaccharide contents were analyzed using the phenol–sulfuric acid method as described by DuBois et al. (1956). The total polyphenolic content was analyzed according to the method described by Chandler and Dodds (1983) The protein content of the CPs was analyzed using the Thermo Scientific Pierce BCA protein assay kit (Thermo Scientific, Waltham, MA, USA). The sulfate content was measured with the BaCl2 gelation method (Dodgson and Price 1962).
Evaluation of antioxidant activities
The antioxidant activity of each CP fraction was analyzed as the measurement of DPPH, alkyl, and hydroxyl free radical scavenging activities. The analysis was done using an electron spin resonance spectrometer (JES-FA200; Jeol, Tokyo, Japan) at 298 K. The DPPH radicle scavenging activity was analysed according to the method described by Nanjo et al. method (Nanjo et al. 1996). The alkyl radical scavenging activity was analyzed according to the method described by Hiramoto et al.’s method (Hiramoto et al. 1993). The hydroxyl radical scavenging activity was evaluated according to the method described by Finkelstein et al. (1980).
Cell culture
“Chang” liver cells were maintained in DMEM supplemented with 1% antibiotic (100 μg mL−1 penicillin and 100 μg mL−1 streptomycin) and 10% FBS. Cell cultures were maintained in incubators provided with a humidified atmosphere at 37°C with 5% CO2. Cells were subcultured within each 2 days and the cells under exponential growth were seeded for experiments. Experiments were carried out using Chang cells seeded in 96 well culture plates following the same methods as described by Wijesinghe et al. (2011). Cytotoxicity of the CPs was evaluated as a measurement of cell viability using MTT colorimetric assay. Readings were obtained using a synergy HT multi-detection microplate reader (BioTek Inc., Winooski, VT, USA).
Evaluation of intracellular ROS scavenging activities
The 2′,7′-dichlorofluorescin diacetate (DCFH-DA) assay was adopted to evaluate the ROS scavenging ability of the CPs as described by Engelmann et al. (2005). Chang cells pre-seeded in 96-well plates at 1.0 × 105 cells mL−1 were treated with different sample concentrations. After 1 h of incubation, H2O2 (1 mM) or 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH; 10 mM) were added to each well except the control. After 1 h of incubation, DCFH-DA (25 μg mL−1) was added to each well following a 10 min incubation period. Fluorescence readings were obtained with a synergy HT multi-detection microplate reader at a 485 nm excitation and 530 nm emission wavelengths.
Characterization of CPs by Fourier transform infrared spectroscopy and neutral sugar analysis
FTIR spectra of the CPs and standard fucoidan, agar, lambda-carrageenan, and alginic acid in KBr pellets were analyzed using an FTIR spectrometer (Nicolet 6700; Thermo Scientific). To analyze neutral sugars CPs were hydrolyzed using 4 M of trifluoroacetic acid in sealed glass tubes. Analysis was carried out using the method described by Kang et al. (2015).
Computational calculations
Infrared (IR) vibrational wave numbers for designed dimeric units of polysaccharides were calculated using the Gaussian 09 package. Initial optimization of the molecular geometry was performed using the PM6 semi-empirical method. Geometry optimization and harmonic vibrational frequencies were calculated using time-dependent density functional theory (DFT) quantum-chemical calculations at the RB3LYP level using the 6-31G(d,p) basis set as described by Cardenas-Jiron et al. (2011). The calculated vibrational spectra were scaled with 0.9645, 0.9799, 0.9819, 0.8625, 0.8719, and 0.9319 for alginic acid, fucoidan, sulfated galactan, mannan, agar, and lambda carrageenan, respectively.
Statistical analysis
All the data values are expressed as mean ± SD based on at least three independent experiments. Statistical analysis for comparing the data was performed using IBM SPSS Statistics 20 software (IBM Corp., Armonk, NY, USA) using one-way ANOVA by Duncan’s multiple range test. p-values less than 0.05 (p < 0.05) were considered as significant.
RESULTS
Proximate composition
As shown in Table 1, J. adhaerens showed the highest ash content followed by the two Halimeda species. The thallus of the aforementioned three algae species was highly calcified. The highest protein content was displayed by C. racemosa var. racemosa f. remota. The highest lipid content was shown by the two G. corticata species. The brown alga C. minima had the highest carbohydrate content followed by the red algae of the three Gracilaria species and A. pygmaea.
Chemical composition
The highest carbohydrate content was shown by SLRS10P with a percentage of 93.83 ± 1.07% followed by SLGS1P with a percentage of 91.45 ± 1.02% (Table 2). The protein content was higher in SLBS11P and SLRS8P. The highest phenolic and sulfate contents were observed in SLBS11P (Table 2).
Radical scavenging activities of CPs
The highest DPPH and alkyl radical scavenging activities were observed in SLBS11P with IC50 values of 89.51 ± 17.00 and 106.80 ± 0.66 μg mL−1, respectively (Table 3). The highest hydroxyl radical scavenging activity was observed in SLGS1P followed by SLBS11P. The antioxidant activity of standard ascorbic acid was measured using each respective radical scavenging assay. Accordingly, IC50 values were 23.22 ± 0.52 μg mL−1 for the DPPH radical scavenging activity, 248.35 ± 0.52 μg mL−1 for the hydroxyl radical scavenging activity and 35.62 ± 0.41 μg mL−1 for the alkyl radical scavenging activity.
Cytotoxicity and protective effects of CPs
None of the CP fractions showed a considerable cytotoxic effect on Chang cells at the concentrations tested (Fig. 1A). Cell viabilities were above 80% even in the presence of the highest concentration (200 μg mL−1). Results of the DCFH-DA assay indicate a reduction in the intracellular ROS levels of Chang cells, induced with H2O2 or AAPH in a dose-dependent manner compared to the respective positive control (Fig. 1B & C). SLBS11P displayed the strongest scavenging effects. In addition to SLBS11P, a robust intracellular H2O2 scavenging activity was observed in SLGS1P (p < 0.001).

Evaluation of sample toxicity and intracellular reactive oxygen species (ROS) scavenging activities of the samples against H2O2 and 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) induced oxidative stress in “Chang” cells. (A) Sample toxicity. (B) AAPH induced intracellular ROS scavenging activity. (C) H2O2 induced intracellular ROS scavenging activity. Results represent the percentage (%) of cell viability and intracellular ROS levels. Values were obtained from three independent experiments and represented as means ± standard deviation. ap < 0.05 and bp < 0.001 were considered as significant compared to the control (sample toxicity) and positive control (ROS scavenging).
Structural analysis
The assignment and characterization of IR vibrational spectra were done based on computational calculations performed on pre-designed monomeric and dimeric units of polysaccharides using the DFT method at the RB3LYP/6-31G(d,p) level and based on relevant literature (Tul’chinsky et al. 1976, Christiaen and Bodard 1983, Mathlouthi and Koenig 1987, Mollet et al. 1998, Roberts and Quemener 1999, Marais and Joseleau 2001, Pereira et al. 2003, 2013, Chandía et al. 2004, Praiboon et al. 2006, Leal et al. 2008, Alves et al. 2010, Ji et al. 2013, Xia et al. 2014). Fig. 2 shows the structures of the constructed dimeric units and their corresponding energy values.
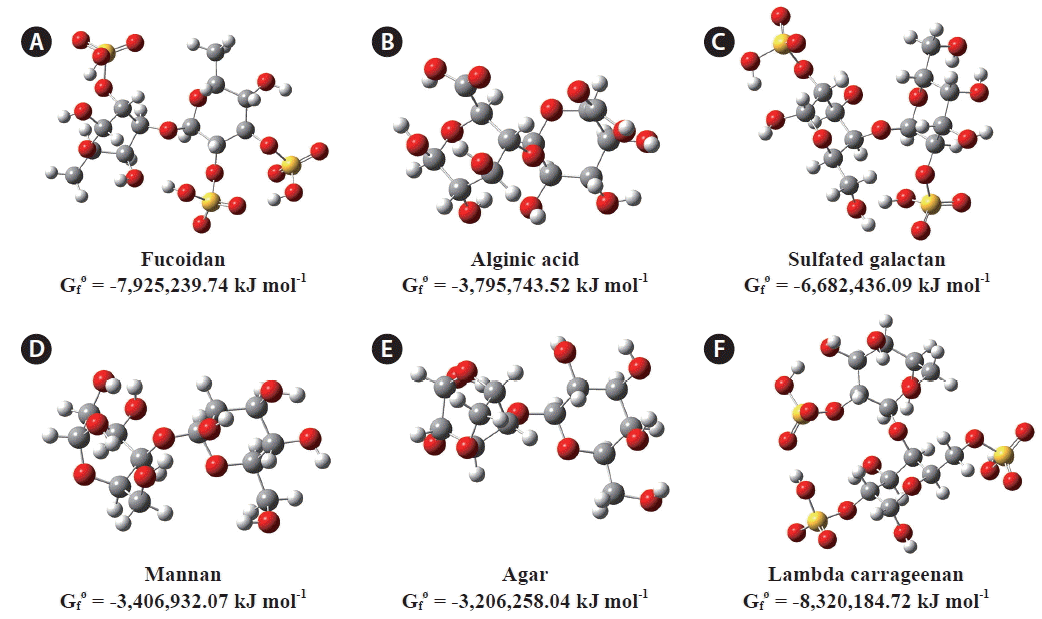
Optimized molecular geometries of the modeled dimeric units of polysaccharide residues. (A) Fucoidan. (B) Alginic acid. (C) Sulfated galactan. (D) Mannan. (E) Agar. (F) Lambda carrageenan. Computational calculations were performed using density functional theory method at RB3LYP/6-31G(d,p) level. Gfø represents the “Sum of electronic and thermal Free energies” (Gibbs free energy) of the molecule in kJ mol−1. Color code for spheres: yellow, sulfur; red, oxygen; blue, nitrogen; grey, carbon; white, hydrogen.
The calculated FTIR spectra are shown in Fig. 3. The IR spectra within the 500 cm−1 to 2,000 cm−1 wavenumber region (fingerprint region for polysaccharides) were used for data analysis. Table 4 summarizes some of the major IR vibrational modes of polysaccharides (Mathlouthi and Koenig 1987, Mollet et al. 1998, Alves et al. 2010, Pereira et al. 2013). All FTIR spectra identify a basic polysaccharide backbone with an intense peak centering 1,035 cm−1 representing the stretching vibrations of the glycoside bridge (C─O─C) (Pereira et al. 2013). This intense peak is broadened (1,010–1,090 cm−1) due to the overlap with of other peaks (Pereira et al. 2003, Xia et al. 2014).
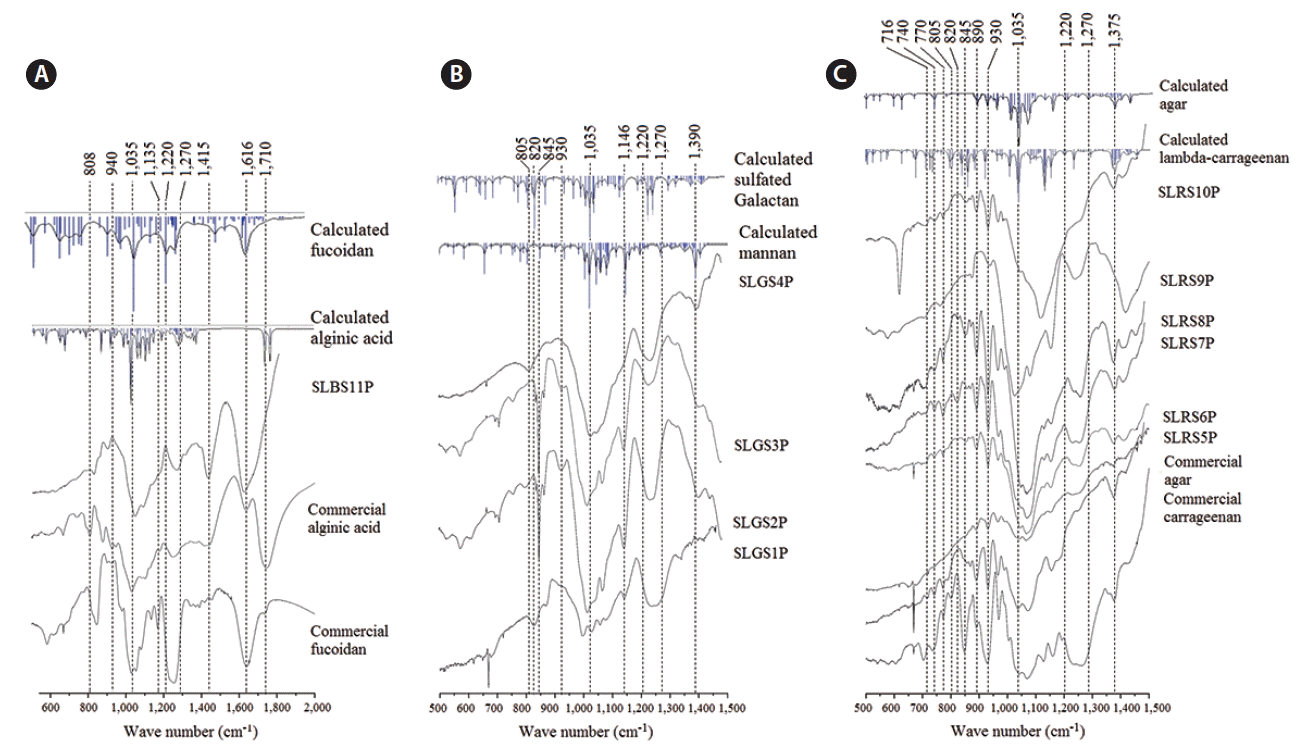
Fourier transform infrared analysis of the crude polysaccharide fractions. (A) Brown algae crude polysaccharides. (B) Green algae crude polysaccharides. (C) Red algae crude polysaccharides. Experimental infrared (IR) spectra of the crude polysaccharide samples have been compared with IR spectra of standard polysaccharides and calculated IR spectra of constructed dimeric units of polysaccharides. Computational calculations were performed using density functional theory at RB3LYP/6-31G(d,p) level.
Considering the polysaccharides found in brown algae, except for the characteristic IR peaks shared by polysaccharides, the band at 1,135 cm−1 (Fig. 3A) in SLBS11P and fucoidan standard indicates stretching vibrations of the glycosidic C─O group of fucoidan. The broadened peak between 1,120–1,270 cm−1 indicate sulfate groups (S═O stretching) branching off from fucoidan or alginic acid residues. The IR band at 845 cm−1, shows the C─O─S bending vibration and further confirms the presence of a sulfate group. The peak at 1,616 cm−1 (Fig. 3A) is originated from the asymmetric stretching vibrations of the carboxylate O─C─O bond. The intense peak at 1,710 cm−1 in the commercial alginic acid indicates a stretching vibration of the carbonyl group in carboxylic acid groups (C═O), and the two peaks at 1,705 and 1,715 cm−1 in calculated alginic acid spectra confirms this feature (Jeon et al. 2002). The 1,710 cm−1 peak was not observed in SLBS11P, since alginic acid is clearly absent from SLBS11P. The absence of IR bands at 808 cm−1 (C1─H deformation vibration of guluronic acid residues), the bands at 808 and 822 cm−1 (guluronic acid residues), and the peak centered at 940 cm−1 (stretching vibration of C─O in uronic acid residues) further confirm this scenario (Chandía et al. 2001, 2004, Leal et al. 2008).
Considering the polysaccharides found in green algae, seaweed galactans have IR peaks at 930, 845, 820, and 805 cm−1 (Fig. 3B) associated with 3,6-anhydrogalactose and the sulfation of C-4, C-6 of galactose units and of C-2 of 3,6-anhydrogalactose (Matsuhiro 1996). The broadened IR peak between 1,120 and 1,270 cm−1 is indicative of the S═O stretching vibration of sulfate groups, which was observed in all the green algal polysaccharide samples. The peak near 1,380 cm−1 is also indicative of sulfate substitution (Fenoradosoa et al. 2009). Peaks at 1,146 and 1,390 cm−1 are due to the presence of mannans (Dunn et al. 2007).
For red algal polysaccharides, the characteristic peak at 930 cm−1 (Fig. 3C) represents the C─O─C vibration of 3,6-anhydro-L-galactose and 3,6-anhydro-D-galactose residues found in both agar and carrageenan. The peak near 1,375 cm−1 indicates the presence of sulfate groups. Peaks at 740 and 716 cm−1 are associated with the C─O─C bending vibration separately in glycosidic linkages. The peak at 890 cm−1 represents the stretching vibration of the anomeric C-H of unsulfated β–galactopyranosyl residues. Based on their degree interaction with sulfates, carrageenans are categorized into several types (Roberts and Quemener 1999). The peak between 1,210 and 1,270 cm−1 is associated with stretching vibration of the S═O bond in sulfate groups, which is generally observable in all carrageenan types. This feature was observed in commercial lambda-carrageenan, SLRS6P, SLRS7P, SLRS8P, and SLRS9P but not SARS10P. According to Roberts and Quemener’s characteristic spectral features can be observed in different carrageenan types (Roberts and Quemener 1999): peaks at 840–850 cm−1 (D-galactose-4-sulfate), at 820–830 cm−1 (D-galactose-2-sulfate), and between 800 and 805 cm−1 (3,6-anhydro-D-galactose-2-sulfate). The weak band observed at 770 cm−1 shows the skeletal bending of galactose rings (Pereira et al. 2003).
Monosaccharide composition of CP fractions
The monosaccharide analysis revealed relatively higher glucose levels in green algae (Table 5), except for SLGS1P that was mainly composed of galactose. Red algae had higher levels of galactose and glucose. Except for SLRS9P, red algae had higher galactose levels than glucose levels. All green and red algal CPs indicated negligible amounts of fucose, whereas the highest reported level of fucose was from the CPs of the SLBS11P brown algae.
DISCUSSION
Marine algae have been widely investigated for their secondary metabolites that possess a wide range of biological activities. Among them algal polysaccharides receive special attention being structurally diversified with decorative functional groups such as sulfate groups. The aim of this study was to extract water soluble CPs from underexplored marine algae harvested from coastal areas of Sri Lanka and to evaluate their antioxidant activities and characterize the structural properties using FTIR analysis.
Being hydrophilic in nature, polysaccharides baring hydroxyl, carboxyl and/or sulfate groups could easily be dissolved in water. The use of hot water to extract algal polysaccharides seems to be a convenient way to enrich CPs. Hydrophilic compounds other than polysaccharides, however cannot be dissolved in water. Ethanol precipitation was therefore employed to precipitate polysaccharides from the mixture by reducing the dielectric constant of the solvent. The yields for the polysaccharides were all higher than 55% (excluding the sulfate attachments) and this was indicative of the reliability of this methodology to obtain CPs. As described by Wijesinghe and Jeon (2012b), however, CPs can form strong intermolecular bonds with phenolic compounds and intra molecular bonds with proteins (glycoproteins). According to literature, the sulfate content and its positioning on the macromolecular backbone of polysaccharides can provide important information to compare their physicochemical characteristics (Wijesinghe and Jeon 2012a). CPs from the brown alga C. minima (SLBS11P) showed comparably high antioxidant activities. The higher levels of sulfate and polyphenols found in the SLBS11P and SLGS1P fractions might have contributed to the antioxidant radical scavenging activities of these CP fractions (Fig. 1).
With quantum chemistry calculation methods, vibrational spectra were obtained for the dimeric units of the major polysaccharides found in algae. The same method has been used by Cardenas-Jiron to compare the FTIR spectra of alginic acid with sample spectra (Cardenas-Jiron et al. 2011). The negative values of “Sum of electronic and thermal free energies” for the constructed dimeric units of polysaccharides suggest stable molecular structures (Fig. 2). The corresponding sample spectra demonstrated the robustness of the DFT approach to predict FTIR spectra (Fig. 3). Alginic acid and fucoidan are the major polysaccharides found in brown algae (Percival 1979). Although alginic acid is the major polysaccharide in brown algae, it’s insolubility in neutral water resulted in the absence of alginic acid from SLBS11P during the extraction procedure. Alginic acid in brown algae consist of D-mannuronic acid and L-guluronic acid. Fucoidan is a SP that mainly contains fucose with varying amounts of galactose, mannose, xylose and glucuronic acid. In addition, fucoidan found in brown algae is widely studied for its biofunctional properties (Wijesekara et al. 2011). Based on FTIR and monosaccharide analysis the polysaccharides obtained from C. minima whereas mainly found to contain fucoidan. Green algae contain highly branched complex molecules of galactans, mannans, and xylans composed of galactose, mannose and xylose units. Moreover, glucuronoxylorhamnan a SP has also been identified in some green algae, and is composed of glucose and rhamnose units (Percival 1979). According to FTIR and monosaccharide analyses SLGS1P, SLGS2P, SLGS3P, and SLGS4P contain galactans and mannans. The major polysaccharides in red algae are galactans that include agar, carrageenan, floridean starch and xylan. Galactose is the major monosaccharide found in red algae that builds up galactans (agar and carrageenan). Moreover, red algae contain floridean starch and xylan composed of glucose and xylose units respectively (Percival 1979). All investigated red algal CPs except SARS10P were characterized by the presence of agar and carrageenan whereas, SARS10P was characterized by abundance of agar.
The chemical mechanism(s) behind polysaccharide antioxidant activity has(have) not systematically been elucidated to date. However, as literature suggest, the higher degree of sulfation in these fucoidans might be attributed to the observed antioxidant activity (Ananthi et al. 2010). Polyphenols bound to polysaccharides might also contribute to the observed antioxidant activity. Further investigation is needed to separate and further purify these polysaccharides and to identify their sequence, monosaccharide composition, and other structural properties.
ACKNOWLEDGEMENTS
This research was a part of the project titled ‘Development of overseas marine bio-resources and a system for their utilization,’ funded by the Ministry of Oceans and Fisheries, Korea. We would also like to thank Department of Wildlife Conservation, Sri Lanka for granting the permission to collect the algae from aforementioned sampling sites.
Abbreviations
AAPH
2,2′-azobis(2-amidinopropane) dihydrochloride
AOAC
Association of Official Analytical Chemists
CP
crude polysaccharide
DCFH-DA
2′,7′-dichlorofluorescin diacetate
DFT
density functional theory
DMEM
Dulbecco’s modified Eagle’s medium
DPPH
2,2-diphenyl-1-picrylhydrazy
FBS
fetal bovine serum
FTIR
Fourier transform infrared
IR
infrared
ROS
reactive oxygen species
SD
standard deviation
SLBS11
Chnoospora minima
SLGS1
Chaetomorpha antennina
SLGS1P
“P” after each sample name denote it’s crude polysaccharide fraction
SLGS2
Halimeda discoidea
SLGS3
Halimeda gracilis
SLGS4
Caulerpa racemosa var. racemosa f. remota
SLRS10
Gracilaria edulis
SLRS5
Gracilaria corticata var. ramalinoides
SLRS6
Gracilaria foliifera
SLRS7
Ahnfeltiopsis pygmaea
SLRS8
Gracilaria corticata
SLRS9
Jania adhaerens
SPs
sulfated polysaccharides




