INTRODUCTION
The global production of cultivated macroalgae more than doubles every decade, and the production of red algae has overtaken brown algae since 2010, dominating the market (Badis et al. 2020b). Production of laver (Pyropia spp., formerly known as Porphyra) (Zuccarello et al. 2022) has also doubled during the same period, primarily due to a rapid expansion in China and Korea, but this rapid development of aquaculture is followed by a similarly increasing economic burden of disease outbreaks (Cottier-Cook et al. 2016, 2021). Disease management is a growing concern for Pyropia farmers, and now contributes up to half the running cost of a farm (Kim et al. 2014). Infected Pyropia are still harvested and used, but crop yield and quality are severely reduced (Klochkova et al. 2017), and market prices may drop by up to one-third of non-infected crops. Infection continues to spread after harvest. The harvested thallus is washed alternately with seawater and fresh water over 2–3 days to remove contaminants, and then goes through a drying process. During this time, the infection spreads further and the dead cell debris promotes bacterial growth, further deteriorating the quality of the final product, but there are no effective and environmentally safe countermeasures to date (Kim et al. 2014).
Various species of fungal, viral, and oomycete pathogens infecting Pyropia crops have been described worldwide (Sekimoto et al. 2008, Kim et al. 2016, Klochkova et al. 2016, Mo et al. 2016, Badis et al. 2018, 2020a). Among them, oomycete pathogens such as Pythium porphyrae and Olpidiopsis spp. cause the greatest damage to Pyropia aquaculture (Kim et al. 2014, Klochkova et al. 2017). Pythium porphyrae, a causative agent of red rot disease, is one of the most serious oomycete pathogen first reported and in Pyropia farms in Asia (Arasaki 1947, Takahashi et al. 1977, Park et al. 2001, Ding and Ma 2005). The geographic distribution of this oomycete includes the Southern Hemisphere where Pyropia plicata in New Zealand was found to be infected with the same species (Diehl et al. 2017). The oomycetes Olpidiopsis porphyrae and O. pyropiae are responsible for the destructive outbreaks of Olpidiopsis-blight in Asia (Sekimoto et al. 2008, Kim et al. 2014, Klochkova et al. 2016, Kwak et al. 2017). Two novel Olpidiopsis species infecting red algae have been reported in Scotland (Badis et al. 2018). A few more species of this genus have been reported to infect other red algae: O. bostrychiae infecting Bostrychia moritziana (West et al. 2006, Sekimoto et al. 2009); O. feldmanni infecting Asparagopsis sp. (Fletcher et al. 2015); and O. heterosiphoniae infecting Dasysiphonia japonica (Klochkova et al. 2017). Investigations into the molecular mechanisms by which these oomycete pathogens infect red algae have only recently begun (Im et al. 2019).
Acid-wash of cultivation nets is the most commonly used measure in Pyropia farms to reduce epiphytic green algae, diatoms, and even oomycete pathogens (Park et al. 2001). A boat equipped with a large tub passes underneath the cultivation net and the net gets immersed in the organic acid in the tub while the boat slowly moves forward. Because of huge area to cover and limited tolerance of Pyropia cells to acidic solution the immersion time should not exceed 30 s. As Pyropia tolerates acidic solutions better than other epiphytic organisms this treatment has been regarded useful to clean the cultivation nets (Park et al. 2001), but this method has little effect on Olpidiopsis blight and is a burden on the environment (Kim et al. 2014). As regulations on aquaculture methods are strengthened, interest in non-acidic treatment is increasing.
Calcium salts has been widely used to control oomycete pathogens in land plants because calcium treatment is known to significantly reduce zoospore production and viability of oomycetes (Brunelli 1995, Nigro et al. 1997, Campanella et al. 2002). It has been reported that the formation of zoosporangia and cleavage of the zoosporangial cytoplasm to zoospores in P. porphyrae is dependent on extracellular calcium and the calcium ion could regulate zoospore release and infectivity of P. porphyrae (Addepalli and Fujita 2002). However, the effectiveness of calcium salts varies greatly depending on the type of crop and pathogen, so it is important to choose the appropriate calcium salt.
In this study, we developed a non-acidic treatment for oomycete diseases in Pyropia farms. We observed the effects of various calcium salts on the progression of oomycete disease and proposed a more effective methods for field practice.
MATERIALS AND METHODS
Isolation and culture of pathogens and host
Pythium porphyrae was isolated from infected blades of Pyropia yezoensis cultivated in Jindo, southwestern seashore of Korea in December 2010 and designated as strain KNU-pyp2. Pythium prophyrae strain was maintained on Arasaki B medium (Arasaki et al. 1968) at 20°C in the dark on agar plates. To obtain zoospores from P. porphyrae, the agar discs of mature fungal hyphae were inoculated into liquid culture medium (Fujita and Zenitani 1977) and grown for 5 days. The zoospores from the liquid culture were induced under axenic conditions following the protocol of Uppalapati and Fujita (2000).
Pyropia yezoensis infected with O. pyropiae were collected from a commercial plantation in Seocheon, Korea. After being transported to the laboratory, blades were cut into approximately 1 × 1 cm pieces and kept in modified Grund medium (MGM) with constant aeration at 10°C under 30 μmol m−2 s−1 light intensity (16 L : 8 D). Stock solution of MGM consisted of C10H14O6N2Na2.2H2O(Na2EDTA) 3.72 g, FeSO4.7H2O 0.28 g, Na2HPO4.12H2O 10.74 g, NaNO3 42.5 g, MnCl2.4H2O 0.019 g, vitamin B12 1 mg, ddH2O 1 L, and was diluted to a concentration of 1 mL per 1 L of filtered autoclaved seawater with salinity of 30 psu (Klochkova et al. 2012). Cultures of Olpidiopsis sp. were constantly maintained for over 10 years by transferring a piece of infected blades to a solution containing healthy Pyropia blades every two weeks.
Pyropia yezoensis was collected from a commercial plantation in Seocheon, and kept in PES medium with constant aeration at 10°C and 30 μmol m−2 s−1 irradiance (16 : 8 h L : D). The medium was replaced every 2 weeks. The cohorts of numerous monospores released from the gametophyte thallus were grown in an environmentally controlled chamber and were used for the infection experiment. Indoor culture strains of Pyropia used in this study have been deposited in culture collection of the National Marine Biodiversity Institute of Korea (MABIK) (No. KNU000212, KNU000216).
Effects of calcium salts on infection of Pythium porphyrae and Olpidiopsis
An inoculum of P. porphyrae and Olpidiopsis zoospores was prepared using stock cultures described above. Healthy blades of P. yezoensis were mixed with oomycete-infected thalli and incubated in seawater containing 10 mM of different calcium salts for 1 h, and the spread of infection was measured after day 2. In order to observe if a brief calcium pretreatment is effective against oomycete infection, Pyropia blades were dipped in a solution containing calcium propionate for 30 s, then transferred to seawater containing the zoospore inoculum, and the progress of infection was measured two days later. The infected area of each experimental group was photographed with a camera attached to Olympus BX51 microscope (Tokyo, Japan), measured with an image analysis program (Image-Pro; Media Cybernetics, Silver Spring, MD, USA), and then compared with the control group (no calcium salt treatment) and calculated as a percentage. All the experiments were carried out three times and each in quadruplicate samples and their standard deviations were calculated.
Field test of calcium propionate treatment
A field test was conducted to observe if the treatment with calcium propionate could inhibit oomycete infection in an aquaculture plantation located in Seocheon, west coast of Korea. A boat equipped with a large tub containing 100 mM calcium propionate in seawater used as per standard practice (see above). Because of large area to cover the immersing time was restricted in 30 s. This calcium propionate wash was done once at a time. Pyropia blades were collected every two weeks and the oomycete-infected areas were measured and compared with nearby control nets treated with acid-wash using the methods described above.
RESULTS
Spread of oomycete infection during laver processing
After harvesting, a large amount of Pyropia is packed into a water tank, transported to a drying facility, and then washed several times with seawater and freshwater for two days. When oomycete-infected thalli are mixed, the infection spreads rapidly during this process which is carried out at room temperature. When we observed the spread of infection over time, oomycetes spread much faster in the tank than in the field (Fig. 1). The infected area, which was initially barely visible, expanded to more than 97% of the thallus within two days in tanks containing thallus infected with O. pyropiae or Pythium prophyrae (Fig. 1).
Effects of calcium salts on the spread of infection
When P. yezoensis thalli infected with P. porphyrae was incubated with 10 mM calcium salt for 1 h in seawater prior to transfer to fresh seawater, the infection area was significantly reduced day 2 after all calcium treatments (Table 1). In particular, in the calcium propionate treatment, it was only 7.07% of the control. The pH of 10 mM calcium propionate dissolved in seawater was 7.88, which was closer toseawater pH than other salts (Table 1). In O. pyropiae infection, the infection-spreading effect of calcium salts was slightly lower than in Pythium prophyrae infection, but calcium propionate still effectively inhibited the spread, which was 10.5% of the control (Fig. 2).
Effects of brief treatment of calcium propionate on the spread of infection
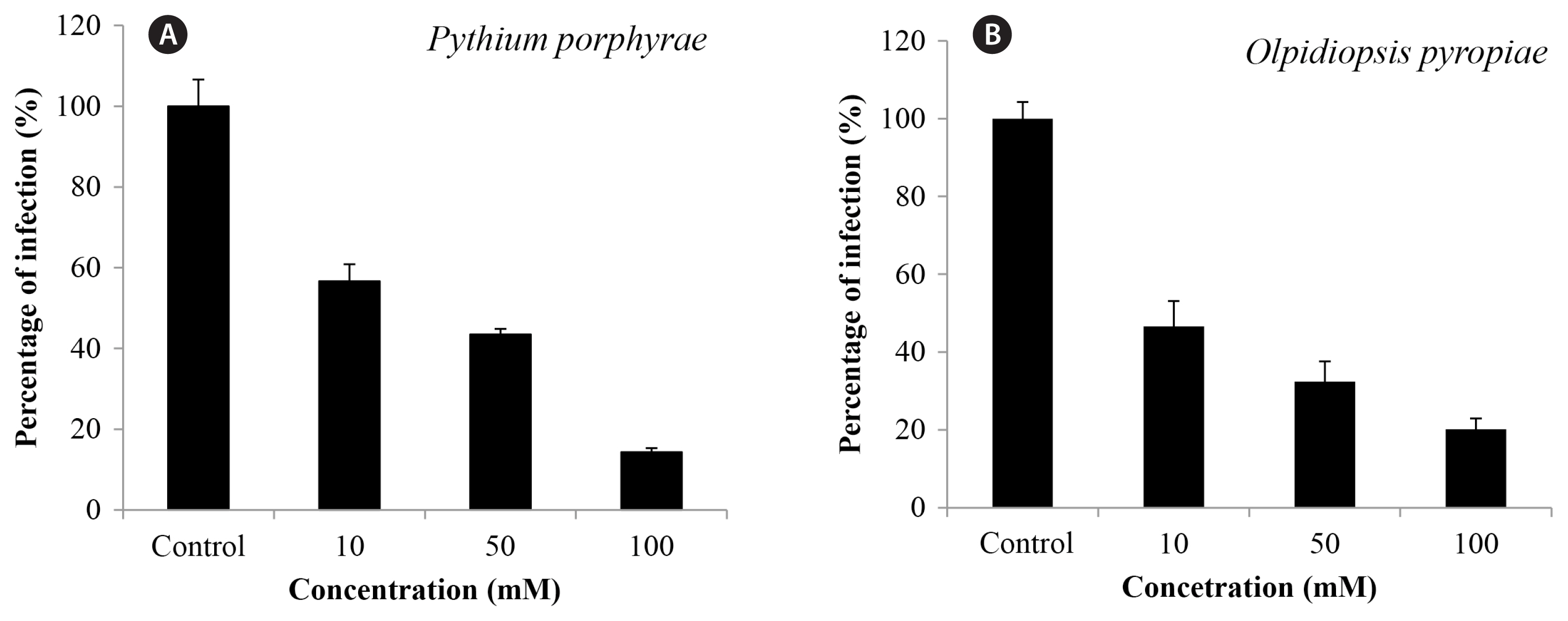
Brief incubation of infected P. yezoensis blades in calcium propionate for 30 s significantly reduced the spread of oomycete infection, but higher concentrations were required compared to 1 h incubation to have a significant effect (Fig. 3). When infected thallus was incubated in 100 mM calcium propionate for 30 s, Pythium prophyrae infection was 14.3% of control and Olpidiopsis pyropieae infection was 20.17% of control (Fig. 3).
Field test of calcium propionate treatment
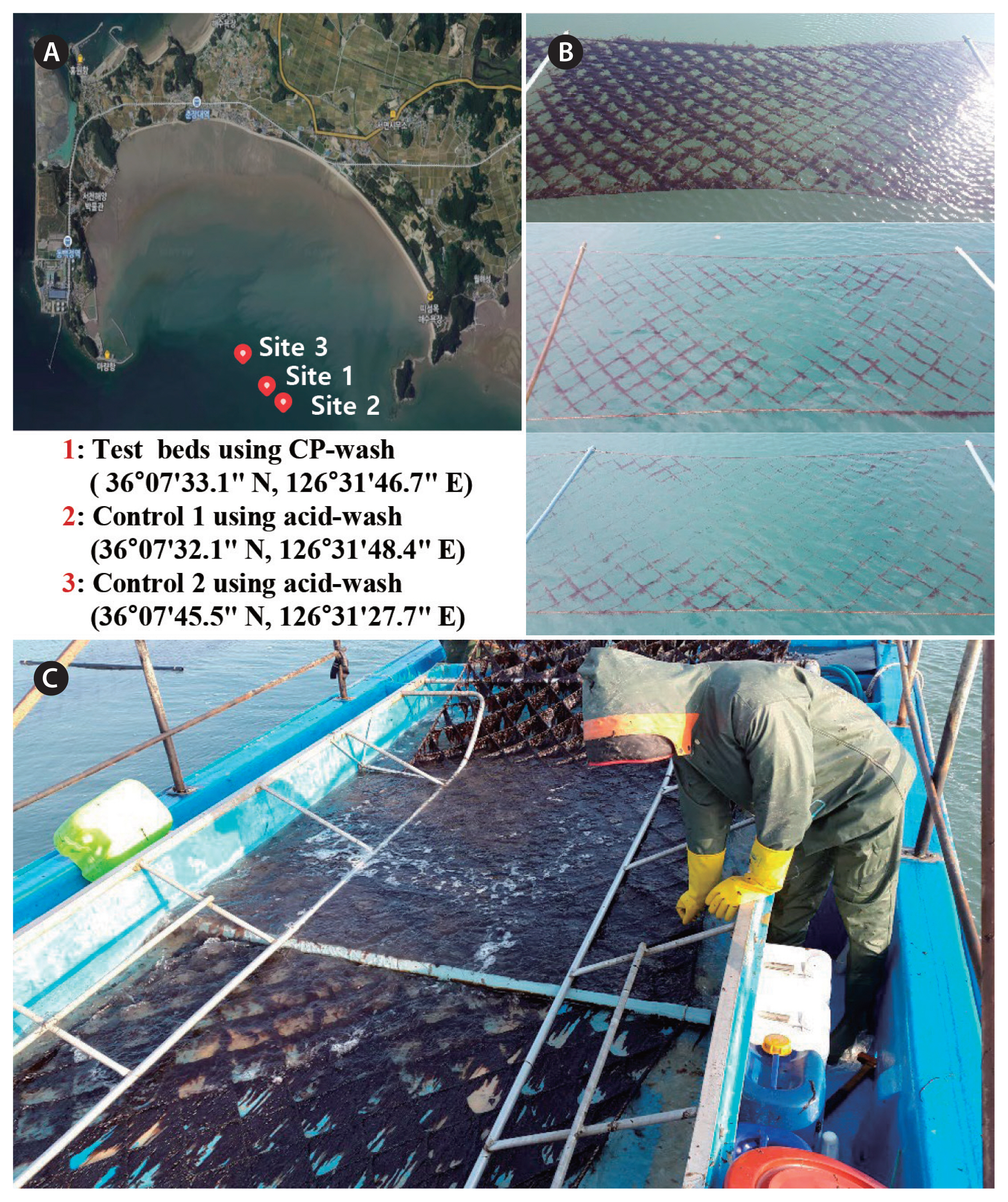
An experiment to control spread of oomycetes in the field using calcium propionate was conducted at an aquafarm in Seocheon, the west coast of Korea, and two control farms located next to the experimental site (Fig. 4A). A boat equipped with a large tub containing 100 mM calcium propionate in seawater was slowly advanced under the net so that the cultivation net was submerged in the solution for 30 s (Fig. 4C). During two seasons in which the field tests were conducted, Olpidiopsis-blight attacked the area and devastated the entire aquaculture farms in the area, but the damage was noticeably less in the cultivation nets treated with calcium propionate (Fig. 4B). During the 2014–2016 seasons Pyropia blades were collected to measure oomycete infection whenever possible (Fig. 5). Red rot disease caused by Pythium pophyrae hit the farm early in the first season and Olpidiopsis blight persisted throughout two seasons, but the infection rates were always lowest in the experimental site treated with calcium propionate (Fig. 5).
Effect of calcium treatment on production volume and market price
The yield and auction prices of P. yezoensis produced in the test farms were obtained from local fisheries cooperatives with the permission of the farmers running the experimental area (Table 2). During two farming seasons, the cultivation nets using calcium propionate treatment earned a minimum of 18.8% and a maximum of 63% more than other nets using acid-wash. Total production decreased by 25–30% in all farms in the second year, but the unit price per kg was higher in the calcium propionate treated areas than in the other two area, so the profit margin was even larger (Table 2).
DISCUSSION
The results of this study show that treatment with calcium propionate can be used as an effective means of controlling the spread of oomycetes during the production and processing stages of Pyropia while improving product quality with a relatively low environmental impact compared to the currently used acid treatment. Olpidiopsis-blight and red rot disease caused by oomycete pathogens are dominant diseases in Pyropia farms in Korea (Cho and Chang 1986, Kim et al. 2014). Oomycete infections not only reduces aquaculture production, but also spread throughout the distribution and processing stages, significantly reducing product competitiveness. Recent estimates from the Korean Ministry of Marine Affairs and Fisheries show that on average, 10% of the annual Pyropia production is lost due to pathogens (Klochkova et al. 2012, Cottier-Cook et al. 2021). Yield and value losses due to Olpidiopsis-blight and red rot disease have been reported as high as 15–20 and 20–30%, respectively (Park et al. 2000), and losses due to oomycete diseases have been reported as high as 77.5% in the past (1983–1985) (Cho and Chang 1986). Our three-year investigation in Pyropia farms showed that currently used acid-wash methods cannot effectively control the infection and spread of oomycetes, especially Olpidiopsis spp. (Kim et al. 2014). Moreover, the transportation and manufacturing processes of Pyropia harvested from farms does not allow for the use of acid treatment.
Government regulations for disease control in South Korea’s seaweed farms are very strict. Chemical treatments are currently limited to a few organic acids or concentrated salts that have been approved through government review and testing in the field (Kim et al. 2014). Farmers also try to avoid chemical treatments as much as possible to reduce operating costs. Pyropia cultivation takes place between October and March, when the water temperature is relatively low in part because the spread of disease is slower during this time. If there is a concern that the water temperature will not drop low enough to prevent the spread of infection other methods can be employed. The freezing net method is sometimes used, in which nets are stored at −20°C and then reinstalled in the field at the appropriate time (e.g., Fujita and Migita 1980, Ding and Ma 2005, Klochkova et al. 2012), but this reduces growth time and is expensive to implement. In some areas, farmers expose aquaculture nets to the air for several hours during the day, taking advantage of the fact that Pyropia thalli are more resistant to cold and desiccation than other organisms (Klochkova et al. 2012). The most common method is acid-washing, which is not effective in preventing the spread of infectious disease (Kim et al. 2014). Despite all these attempts, oomycete pathogens are still the biggest problem for Pyropia farms. This study shows that calcium propionate salts are very useful in controlling oomycete pathogens, although government regulations need to be modified before this method can be used more broadly. However, calcium propionate treatment can be a useful means of effectively preventing the spread of infection during the transportation and processing step of harvested Pyropia, even without legislative changes.
Calcium salt treatments are widely used to eliminate oomycetes that cause root/fruit rot in many important crops including citrus, soybeans, potatoes, onions, and apples, and have low environmental impact (Kaiser et al. 2011, Türkkan 2013). The inhibitory effects of calcium salts on oomycete pathogens include reduced germ tube growth, reduced mycelial growth in vitro, and reduced severity of infection of pretreated host tissues (Biggs et al. 1997, Campanella et al. 2002). While the types of calcium salts applied to different crops vary greatly, calcium propionate is one of the most effective calcium salts against oomycete pathogens on a wide variety of terrestrial crops (Kaiser et al. 2011). Calcium propionate has been used for decades as a food additive to inhibit mold in bakery products (Furia 1973). It also occurs naturally in butter and some types of cheese and is regarded as relatively safer than other food additives by the European Food Safety Authority (EFSA Panel on Food Additives and Nutrient Sources Added to Food 2014).
The regulatory role of external calcium on zoospore release, development, and infection of P. porphyrae has been studied, using EGTA to inhibit the influx of external calcium, but the regulatory role of calcium salts has not been studied (Addepalli and Fujita 2002). Calcium (Ca2+) is a universal cell signaling molecule involved in myriad biological responses in a variety of organisms (Zhang et al. 2014, Aldon et al. 2018), but little is known about why calcium salts inhibit oomycete reproduction and infection to a much greater extent than their hosts. To develop more effective treatments for oomycete pathogens, research into calcium signaling during oomycete infection is needed.
This study showed that treatment with 100 mM calcium propionate for 30 s using the same method used by fishermen for acid-washing can effectively control oomycetes in Pyropia aquaculture, but aquaculture is regulated by much stricter regulations than land-based crop cultivation, this method cannot be immediately applied in field practice, except in test farms, under the current legal system. In the future, efforts should be made to revise legislation and educate fishermen to ensure more effective and environmentally-friendly control of oomycetes can be utilized.