ABSTRACT
Cochlodinium polykrikoides red tides have caused great economic losses in the aquaculture industry in many countries. To investigate the roles of metazooplankton in red tide dynamics of C. polykrikoides in the South Sea of Korea, the abundance of metazooplankton was measured at 60 stations over 1- or 2-week intervals from May to November 2014. In addition, the grazing impacts of dominant metazooplankton on red tide species and their potential heterotrophic protistan grazers were estimated by combining field data on the abundance of red tide species, heterotrophic protist grazers, and dominant metazooplankton with data obtained from the literature concerning ingestion rates of the grazers on red tide species and heterotrophic protists. The mean abundance of total metazooplankton at each sampling time during the study was 297–1,119 individuals m−3. The abundance of total metazooplankton was significantly positively correlated with that of phototrophic dinoflagellates (p < 0.01), but it was not significantly correlated with water temperature, salinity, and the abundance of diatoms, euglenophytes, cryptophytes, heterotrophic dinoflagellates, tintinnid ciliates, and naked ciliates (p > 0.1). Thus, dinoflagellate red tides may support high abundance of total metazooplankton. Copepods dominated metazooplankton assemblages at all sampling times except from Jul 11 to Aug 6 when cladocerans and hydrozoans dominated. The calculated maximum grazing coefficients attributable to calanoid copepods on C. polykrikoides and Prorocentrum spp. were 0.018 and 0.029 d−1, respectively. Therefore, calanoid copepods may not control populations of C. polykrikoides or Prorocentrum spp. Furthermore, the maximum grazing coefficients attributable to calanoid copepods on the heterotrophic dinoflagellates Polykrikos spp. and Gyrodinium spp., which were grazers on C. polykrikoides and Prorocentrum spp., respectively, were 0.008 and 0.047 d−1, respectively. Therefore, calanoid copepods may not reduce grazing impact by these heterotrophic dinoflagellate grazers on populations of the red tide dinoflagellates.
INTRODUCTIONMetazooplankton including copepods, cladocerans, chaetognaths, larvae of invertebrates, and hydrozoans are a major component of the marine ecosystem (Fulton 1984, Kang et al. 1996, Uye and Liang 1998, Calbet 2001, Gallienne and Robins 2001, Puelles et al. 2003, Kimmel and Roman 2004, Turner 2004, Tseng et al. 2009). They are consumers of phytoplankton and heterotrophic protists and are in turn prey for larval fish and other metazoans (Porter et al. 1985, Houde and Roman 1987, Turner et al. 1988, Stoecker and Capuzzo 1990, Sanders and Wickham 1993, Carlsson et al. 1995, Croll et al. 2005, Jeong et al. 2007, Waggett et al. 2008, Lazareva and Kopylov 2011). Thus, the dynamics of metazooplankton affect the population dynamics of diverse marine organisms.
Red tides or harmful algal blooms are caused by exclusively autotrophic, mixotrophic, and heterotrophic protists (Smayda 1997, Jeong et al. 2010, 2013, 2015, Lee et al. 2015, Yoo et al. 2015, Aktan and Keskin 2017) and often cause great economic losses in the aquaculture and tourism industries (Smayda 1990, Glibert et al. 2005, Anderson et al. 2012, Fu et al. 2012). Thus, understanding the processes of red tides and predicting their outbreak, persistence, and decline are needed to minimize loss (e.g., Jeong et al. 2015). There have been many studies on the feeding of metazooplankton on red tide organisms, but fewer studies on the grazing impacts of metazooplankton on red tide organisms (Uye 1986, Turner and Granéli 1992, Turner and Tester 1997, Calbet et al. 2003). Thus, it is worthwhile to explore the effects of metazooplankton on red tide organisms in natural environments. In general, many heterotrophic protists are effective grazers on red tide organisms and are in turn prey for metazooplankton (Houde and Roman 1987, Jeong and Latz 1994, Carlsson et al. 1995, Jeong 1999, Jeong et al. 1999, 2001, 2003, Kim and Jeong 2004, Tillmann 2004, Cohen et al. 2007, Yoo et al. 2010, 2013b, Park et al. 2013). An assessment of predation impacts of metazooplankton on populations of heterotrophic protists that graze on red tide organisms is needed to understand the dynamics and interactions among these three components.
Red tides frequently occur in the South Sea of Korea where there is a high concentration of aquacages (Jeong et al. 2013, 2017, Kim et al. 2013a, Lim et al. 2017). These red tides often cause a great loss in the aquaculture industry (e.g., Park et al. 2013). The causative species for red tides in this region are Cochlodinium polykrikoides, Prorocentrum spp., Alexandrium spp., Ceratium spp., Karenia spp., and diatoms (e.g., Jeong et al. 2017). In particular, red tides dominated by C. polykrikoides have caused 1–60 M US dollars-worth of damage (Park et al. 2013). Thus, predicting the process of red tides is a critical step in minimizing damage. There have been several studies on the effects of physical, and chemical, biological environmental factors on the growth of C. polykrikoides (Jeong et al. 2004, 2008, 2017, Kim et al. 2004, Oh et al. 2006, Lim et al. 2014, 2017). However, few studies have investigated the grazing impact by metazooplankton on C. polykrikoides (Kim 2005, Kim et al. 2013a). Therefore, it is worthwhile to explore this topic.
In the present study, the abundance of metazooplankton was measured at 60 stations over 1- or 2-week intervals, from May to November 2014, to investigate the roles of metazooplankton in the red tide dynamics of C. polykrikoides in the South Sea of Korea. In addition, the grazing impacts of dominant metazooplankton on red tide species and their potential heterotrophic protistan grazers were estimated by combining field data on the abundance of red tide species, heterotrophic protist grazers, and dominant metazooplankton with data obtained from the literature concerning ingestion rates of the grazers on red tide species and heterotrophic protists. The results of the present study provide a basis for understanding the interactions among red tide organisms, heterotrophic protists, and metazooplankton, and help understand the roles of metazooplankton in red tide dynamics.
MATERIALS AND METHODSStudy areaPlankton samples were collected from 60 sampling stations located centrally along the southern coast of the Korean peninsula between Goheung to Tongyoung (approximately 140 km × 40 km, 34.3° E-34.8° E and 127.3° N-128.4° N) (Fig. 1). The water depth for all sampling stations ranged from 4–53 m. The stations in the western area were shallower than those in the eastern area at the same latitude.
Sampling and analyses of physicochemical and biological propertiesSixteen sampling cruises were carried out at 1–2-week intervals between May 7 and Nov 10, 2014 (Fig. 2). A 2-day sampling was carried out by two ships as in Jeong et al. (2017) and Lim et al. (2017). On the first day, water samples were collected from one ship at stations 101–115, whereas the other ship collected samples at stations 201–309. On the second day, one ship collected water samples at stations 501–507 and 601–608, whereas the other ship collected at stations 701–703, 801–805, and 901–904. The sampling time at the first station in each sampling day was between 07:30–08:00 h and at the last station between 14:00–15:00 h.
Water temperature and salinity in the water column were measured using two CTDs (YSI6600; YSI Inc., Yellow Springs, OH, USA and Ocean seven; Idronaut S.r.l., Milan, Italy). The data obtained from the CTDs were calibrated in each cruise. Additionally, the temperature, salinity, pH, and dissolved oxygen (DO) for each sampling depth were measured using a YSI Professional Plus instrument (YSI Inc.). The chlorophyll-a concentration (Chl-a) was measured as described in American Public Health Association (1995).
Metazooplankton samples were collected at every station by towing a 303 μm mesh, 45 cm diameter conical plankton net with a flowmeter vertically from the bottom to the surface (or 30 m if the bottom depth was more than 30 m) every sampling interval from May to November 2014. Each plankton sample was poured into a 500-mL polyethylene bottle and preserved with 4% formalin. Species identification and determination of metazooplankton abundance was performed using dissecting and inverted microscopes at magnifications of ×40 and ×200.
Data on phytoplankton, including red tide species and heterotrophic protists, were obtained from Jeong et al. (2017) and Lim et al. (2017) in which sampling was conducted at the same times and stations as in this study.
Grazing impactThe calanoid copepods Acartia spp. are known to feed on various species of dinoflagellates such as Cochlodinium polykrikoides, Prorocentrum donghaiense, Polykrikos kofoidii, and Oxyrrhis marina (Jeong et al. 2001, Kim 2005). Thus, it was assumed that the ingestion rate of Acartia spp. on dinoflagellates C. polykrikoides, P. donghaiense, P. kofoidii, and O. marina were the same as the ingestion rate of total calanoid copepods on the C. polykrikoides, Prorocentrum spp., P. kofoidii, and Gyrodinium spp., respectively.
The grazing coefficients (g, d−1) were calculated as
where CR (mL predator−1 h−1) is the clearance rate of the predator on a target prey at a prey concentration and PC is a predator concentration (predator mL−1). The CR values were calculated as
where IR (cells eaten predator−1 h−1) is the ingestion rate of a predator on the target prey and X (cells mL−1) is the prey concentration. These CR values were corrected using Q10 = 3.2 (Hansen et al. 1997) because the in-situ water temperature and the temperature used in the laboratory for this experiment (20–22°C) were sometimes different.
Data processThe spatiotemporal distributions of each taxon of metazooplankton communities were plotted by Surfer (Golden software, LLC, Golden, CO, USA). The correlation coefficients between physical, chemical, and biological properties were calculated using the Pearson’s correlation (Conover 1980, Zar 1999). By combining field data on the abundance of predator and prey species with the ingestion rates of the predator on the prey obtained in the literatures with some assumptions, we estimated the grazing coefficients.
RESULTSEnvironmental propertiesThe mean surface water temperature during the study varied from 15.6 to 24.7°C with the highest temperature occurring in August 2014 and the lowest temperature in May (Fig. 2A). The mean surface salinity ranged from 31.4 to 34.1 with the highest salinity occurring in May and the lowest salinity in August (Fig. 2B). The pH ranged from 8.02 to 8.22 and the Chl-a from 1.0 to 3.6 μg L−1 with a peak on Sep 1 (Fig. 2C & D). DO ranged from 7.2 to 8.3 mg L−1 (Fig. 2E).
Species composition of metazooplanktonDuring the study 23 copepods, 4 cladocerans, 10 invertebrate larva, and 10 other metazooplankton taxa were found (Appendix 1). These included the copepods Acartia erythraea, A. omorii/hongi, Calanopia thompsoni, Calanus sinicus, Centropages abdominalis, Centropages tenuiremis, Corycaeus affinis, Euchaeta sp., Labidocera euchaeta, Labidocera rounta, Labidocera sp., Neocalanus sp., Oithona similis, Oithona sp., Oncaea sp., Paracalanus parvus, Pseudodiaptomus marinus, Sinocalanus tenellus, Temora turbinata, Temora discaudata, Tortanus forcipatus, harpacticoid copepods, and copepodites, the cladocerans Evadne nordmanni, E. tergestina, Penilia avirostris, and Podon polyphemoides. In addition, barnacle larvae, decapod zoea, chaetognaths, siphonophores, and appendicularians were observed.
Of these metazooplankton, A. omorii/hongi, C. affinis, barnacle nauplii, decapod zoea, decapod mysid, echinoderm larvae, gastropod larvae, polychaete larvae, chaetognaths, hydromedusa, siphonophores, amphipods, and appendicularians were present at all sampling times (Appendix 1). However, C. abdominalis, Neocalanus sp., S. tenellus, and E. nordmanni were only present during one sampling.
Abundance of metazooplanktonThe abundance of total metazooplankton at each sampling during this study were 1–13,131 individuals (inds.) m−3 and mean abundance was 297–1,119 inds. m−3 (Table 1, Fig. 3). The mean abundance of total metazooplankton was high from Jun 23 to Aug 13 (Fig. 3B).
Copepods dominated metazooplankton assemblages at all sampling times except from Jul 11 to Aug 6 when cladocerans and hydrozoans dominated (Fig. 3). The maximum mean abundance of copepods was 453 inds. m−3 on May 21. Furthermore, cladocerans and benthic invertebrate larvae dominated metazooplankton assemblages on Jun 23 with the maximum mean abundance of cladocerans and benthic invertebrate larvae being 274 and 178 inds. m−3, respectively (Fig. 3). Moreover, chaetognaths, hydrozoans, and other metazooplankton taxa were abundant from Jun 23 to Aug 13 (Fig. 3). The maximum mean abundance of chaetognaths, hydrozoans, and other metazooplankton was 87, 210, and 178 inds. m−3, observed on Sep 28, Jul 22, and Jun 23, respectively (Fig. 3).
Spatiotemporal distributions of the total metazooplanktonMetazooplankton predominantly inhabited the marginal and shallower regions (<30 m depth) of the study area. From May 7 to Aug 13, metazooplankton occurred in high concentrations (<13,200 inds. m−3) near the shallow waters of Goheung and Yeosu (Fig. 4). In contrast, on Sep 1, metazooplankton were regionally concentrated (<2,700 inds. m−3) in the shallow waters of Tongyoung, and abundance of total metazooplankton was high (<1,800 inds. m−3) in the shallow waters of Goheung from Sep 15 to 27 (Fig. 4). Furthermore, metazooplankton were distinctively more concentrated (<1,900 inds. m−3) in the offshore region (>30 m depth) than the shallow waters in November (Fig. 4). In general, the distributions of total copepods and larvae of invertebrates were similar to that of total metazooplankton (Figs 4 & 5).
Correlations between abundance of major metazooplankton taxa and environmental factorsThe abundance of total metazooplankton was significantly positively correlated with the concentration of total phototrophic dinoflagellates, but negatively correlated with pH (Table 2). The abundance of copepods was significantly positively correlated with salinity, but negatively correlated with water temperature (T), pH, and DO (Table 2). Moreover, the abundance of cladocerans was significantly positively correlated with DO (Table 2).
The abundance of invertebrate larvae was significantly positively correlated with T, Chl-a, and abundance of phototrophic dinoflagellates and tintinnid ciliates (TCI) but negatively correlated with salinity and pH (Table 2). Furthermore, the abundance of the chaetognaths was significantly positively correlated with T, but negatively correlated with salinity, pH, and DO (Table 2). However, the abundance of chaetognaths significantly positively correlated with Chl-a and TCI (Table 2). In addition, the abundance of the hydrozoans was significantly positively correlated with temperature, but negatively correlated with pH (Table 2).
At the species level, the abundance of C. sinicus, echinoderm larvae, and siphonophores was significantly positively correlated with the abundance of total diatoms, whereas the abundance of C. affinis, barnacle nauplii, decapod zoea, echinoderm larvae, fish larvae, and siphonophores was significantly positively correlated with the abundance of the diatom Pseudo-nitzschia spp. (Table 3). Furthermore, the abundance of C. affinis, L. euchaeta, L. rotunda, barnacle nauplii, decapod zoea, fish larvae, hydromedusa and siphonophores was significantly positively correlated with that of total phototrophic dinoflagellate (Table 4). In addition, the abundance of C. affinis, L. euchaeta, L. rotunda, P. parvus, P. avirostris, barnacle nauplii, decapod zoea, fish larvae, chaetognaths, hydromedusa, and siphonophores was significantly positively correlated with that of the dinoflagellate P. donghaiense (Table 4). The abundance of C. affinis, L. euchaeta, L. rotunda, P. parvus, copepodite, P. avirostris, barnacle nauplii, decapod zoea, echinoderm larvae, fish larvae, chaetognaths, hydromedusa, and siphonophores was significantly positively correlated with that of the tintinnid ciliate Tintinnopsis tubulosoides (Table 5). Finally, the abundance of salpids was significantly negatively correlated with that of T. tubulosoides (Table 5).
Grazing impact by calanoid copepods on red-tide organisms and heterotrophic dinoflagellatesWhen the abundance of the phototrophic dinoflagellate C. polykrikoides and co-occurring calanoid copepods was 1–2,990 cells mL−1 and 1–2,480 inds. m−3, respectively, the calculated grazing coefficient attributable to calanoid copepods on co-occurring C. polykrikoides was up to 0.018 d−1 (Jeong et al. 2017) (Fig. 6). Furthermore, when the abundance of the phototrophic dinoflagellate Prorocentrum spp. and co-occurring calanoid copepod species was 0.2–2,725 cells mL−1 and 0.9–1,076 inds. m−3, respectively, the calculated grazing coefficients attributable to total calanoids on co-occurring Prorocentrum spp. were 0.001–0.029 d−1 (Jeong et al. 2017) (Fig. 7).
When the abundance of the heterotrophic dinoflagellates Gyrodinium spp. and co-occurring calanoid copepods was up to 42 cells mL−1 and 2,940 inds. m−3, respectively, the calculated grazing coefficients attributable to calanoid copepods on co-occurring Gyrodinium spp. were 0.0003–0.047 d−1 (Lim et al. 2017) (Fig. 8). When the abundance of the heterotrophic dinoflagellates Polykrikos spp. and co-occurring calanoid copepods was 0.02–79 cells mL−1 and up to 1,035 inds. m−3, respectively, the calculated grazing coefficients attributable to calanoid copepods on co-occurring Polykrikos spp. were 0.0001–0.008 d−1 (Lim et al. 2017) (Fig. 9).
DISCUSSIONAbundance of metazooplanktonThe maximum abundance of total metazooplankton obtained during this study (1.3 × 104 inds. m−3) is comparable to that in Jinhae Bay, Kangjin Bay, Seomjin River Estuary, and coastal waters of Yeosu which are located in the South Sea of Korea and Balearic Sea of Mallorca, but slightly lower than that in the Masan Bay (Korea), Newport River Estuary (UK), and NW Mediterranean (Table 6). Chl-a concentrations in the water from which the maximum abundance of total metazooplankton was obtained (2.4 μg L−1) is also comparable to that in the Seomjin River Estuary, Yeosu, NW Mediterranean, and the Balearic Sea of Mallorca, but much lower than that in Masan Bay (Calbet et al. 2001, Puelles et al. 2003, Youn et al. 2010, Kim et al. 2013a, Oh et al. 2013). Thus, in general, the maximum abundance of total metazooplankton is likely to be affected by Chl-a. Furthermore, the maximum abundance of total copepods during this study (0.4 × 104 inds. m−3) is comparable to or slightly lower than that in Kangjin Bay, coastal waters of Yeosu, the NW Mediterranean, and the Balearic Sea of Mallorca, but considerably lower than that in Masan Bay, Fukuyama Harbor (Japan), the NE Atlantic Ocean, and the Pearl River Estuary (China) (Table 6). Chl-a concentrations coinciding with the maximum abundance of copepods were much lower than that in Masan Bay and Fukuyama Harbor (Uye and Liang 1998, Kim et al. 2013a). Thus, the lower Chl-a in this study may be partially responsible for the lower maximum abundance of copepods.
Effect of environmental factors on the abundance of metazooplankton taxaDuring this study, the abundance of total metazooplankton was not significantly affected by T, S, DO, and the concentrations of NO3, PO4, and SiO2. Furthermore, it was also not significantly affected by the abundance of diatoms, euglenophytes, cryptophytes, heterotrophic dinoflagellates, tintinnid ciliates, and naked ciliates. However, it was significantly affected by the abundance of phototrophic dinoflagellates. During this study, four phototrophic dinoflagellate species such as P. donghaiense, C. furca, A. fraterculus, and C. polykrikoides formed red tides (Jeong et al. 2017). Thus, dinoflagellate red tides may positively affect the abundance of total metazooplankton (Griffin et al. 2001, Turner and Borkman 2005, Jansen et al. 2006, Kim et al. 2013a).
During this study, P. donghaiense formed red tides from June to July (Jeong et al. 2017). The abundance of the copepods C. affinis, L. euchaeta, L. rotunda, P. parvus, barnacle nauplii, decapod zoea, fish larvae, chaetognaths, hydromedusa, siphonophores, and the cladoceran P. avirostris is significantly positively correlated with that of P. donghaiense. However, there have been no studies on feeding by these metazooplankton taxa on P. donghaiense and thus, it is worthwhile to explore this topic. During this study, Ceratium spp. formed red tides from July to August (Jeong et al. 2017). The abundance of the cladocerans E. tergestina and P. avirostris, barnacle nauplii, and echinoderm larvae is significantly positively correlated with that of two Ceratium species. The cladocerans E. nordmanni, P. avirostris, and Podon intermedius are known to feed on diverse algal species including Ceratium spp. (Katechakis and Stibor 2004). However, there have been no studies measuring ingestion rates of these metazooplankton taxa on Ceratium spp. and thus it is worthwhile to investigate.
Grazing impact by dominant metazooplankton on red tide organismsIn this study, calanoid copepods dominated metazooplankton assemblages at most sampling times. The calculated grazing coefficients attributable to calanoid copepods on co-occurring C. polykrikoides were up to 0.018 d−1 (i.e., up to 1.8% of the population of C. polykrikoides was removed by calanoids in a day). Furthermore, the calculated grazing coefficients attributable to calanoid copepods on co-occurring Prorocentrum spp. were up to 0.029 d−1 (i.e., up to 2.9% of the population of Prorocentrum spp. was removed by calanoid copepods in a day). Therefore, calanoid copepods may not control populations of C. polykrikoides or Prorocentrum spp. Furthermore, this maximum grazing coefficient is much lower than that by heterotrophic dinoflagellates and ciliates (Lim et al. 2017). In Masan Bay in 2004–2005, the grazing impact by the dominant calanoid copepod Acartia spp. on populations of Prorocentrum minimum was also much lower than that by the heterotrophic dinoflagellates and ciliates (Kim et al. 2013a, Yoo et al. 2013a). The abundance of copepod grazers is usually much lower than that of heterotrophic protist grazers although the ingestion rates of the former are greater than those of the latter. Thus, lower copepod abundance may be partially responsible for a lower grazing impact on populations of red tide dinoflagellates.
The calculated grazing coefficients attributable to total calanoid copepods on the co-occurring heterotrophic dinoflagellates Gyrodinium spp. were up to 0.047 d−1 (i.e., up to 4.6% of the population of Gyrodinium spp. were removed by total calanoids in a day). Furthermore, the calculated grazing coefficients attributable to total calanoid copepods on co-occurring Polykrikos spp. were up to 0.008 d−1 (i.e., up to 0.8% of the population of Polykrikos spp. were removed by total calanoids in a day). Therefore, calanoid copepods may not control populations of Gyrodinium spp. and Polykrikos spp.
During this study, the heterotrophic dinoflagellates Gyrodinium spp. were abundant during the red tides dominated by P. donghaiense (Jeong et al. 2017, Lim et al. 2017). Furthermore, the calculated grazing coefficients attributable to Gyrodinium dominans/G. moestrupii on co-occurring P. donghaiense were up to 0.58 d−1 (i.e., up to 44% of the population P. donghaiense were consumed in 1 d). Therefore, populations of P. donghaiense might be affected by the grazing of Gyrodinium spp., but the grazing impact could be lowered by the predation of calanoid copepods. Moreover, C. polykrikoides populations were affected by grazing of Polykrikos spp. (Lim et al. 2017). However, the grazing impact may not be lowered by predation of calanoid copepods.
Red tides have occurred in coastal waters of many countries (Holmes et al. 1967, Hallegraeff 1993, Anderson 1997, Sordo et al. 2001, Kang et al. 2013, Jeong et al. 2017). The results of this study suggest that red tides could affect the abundance of metazooplankton and in turn the grazing impact by metazooplankton could sometimes affect the abundance of red tide organisms in the South Sea of Korea. Thus, to understand interactions among red-tide organisms, heterotrophic protists, and metazooplankton, and the roles of heterotrophic protists and metazooplankton in the dynamics of red-tide organisms, the temporal and spatial variations in their distributions should be simultaneously investigated. Additionally, the grazing impact by heterotrophic protists and metazooplankton on populations of red tide organisms and in turn the predation impact on populations of heterotrophic protists should be quantified.
ACKNOWLEDGEMENTSWe thank Sang Pil Han, Seong Uk Kim, Young Seok Rho, Cheol Hee Kang for technical support. This research was supported by the National Research Foundation (NRF) funded by the Ministry of Science and ICT (NRF-2015M1A5A1041806; NRF-2017R1E1A1A01074419) and Pilot project for predicting the outbreak of Cochlodinium polykrikoides red tides funded by MSI (NRF-2014M4A1H5009428), the Useful Dinoflagellate Program of Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (MOF) and Management of marine organisms causing ecological disturbance and harmful effect Program of KIMST, and Research Institute of Oceanography, SNU award to HJJ.
Fig. 1Map of the study area (A) and sampling stations (B) in the South Sea, Korea. (B) Section enlarged from map (A). 
Fig. 2Environmental factors in the South Sea of Korea from May 7 to Nov 10, 2014. (A) Average water temperature (T, °C). (B) Average salinity (S). (C) pH. (D) Average chlorophyll-a concentrations (Chl-a, mg m−3). (E) Dissolved oxygen (DO, mg L−1). Symbols represent average ± standard error. 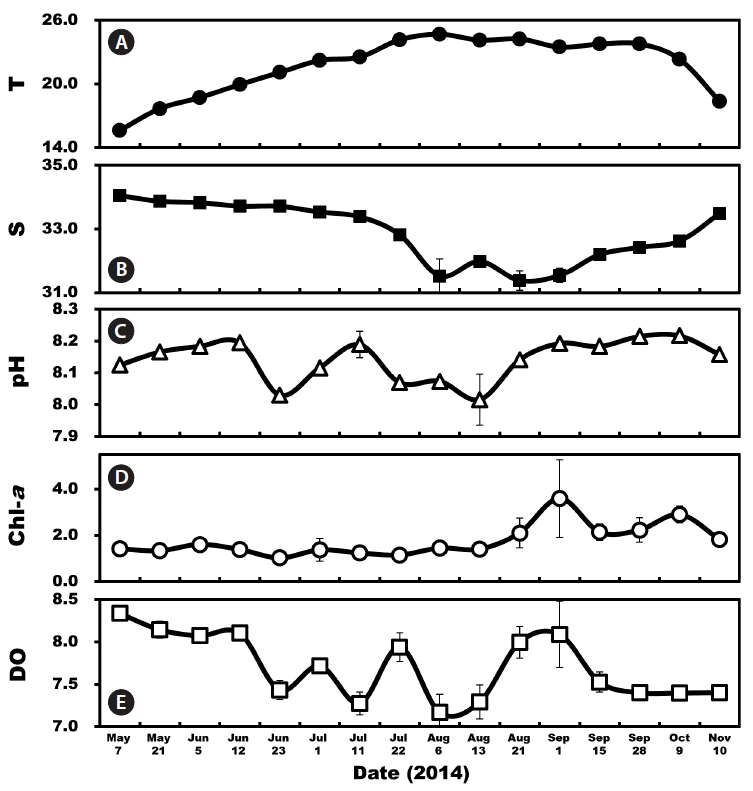
Fig. 3The period of red tides and abundance (inds. m−3) of metazooplankton taxa. (A) The period of red tides dominated by diatoms (Dia), Prorocentrum donghaiense (Pd), Ceratium furca (Cf), Alexandrium fraterculus (Af), and Cochlodinium polykrikoides (Cp). Average abundance of total metazooplankton (B), total copepods (C), total cladocerans (D), larvae of invertebrates (E), chaetognaths (F), hydrozoans (G), and others (H). Bars represent average ± standard error. 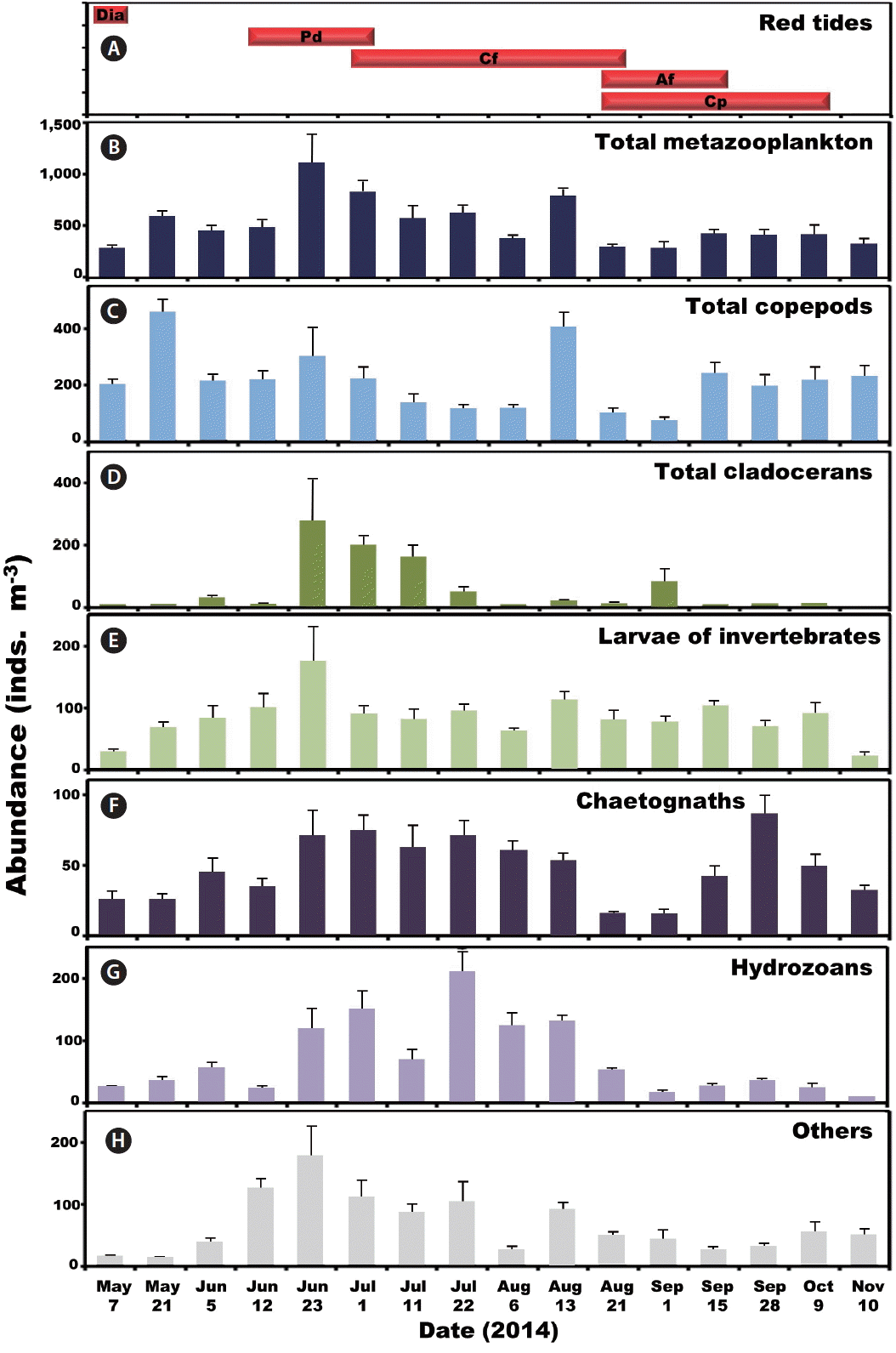
Fig. 4Distributions (inds. m−3) of total metazooplankton (TMZ), total copepods (TCop), and total cladocerans (TCla) in the study area for each cruise from May 7 to Nov 10, 2014. 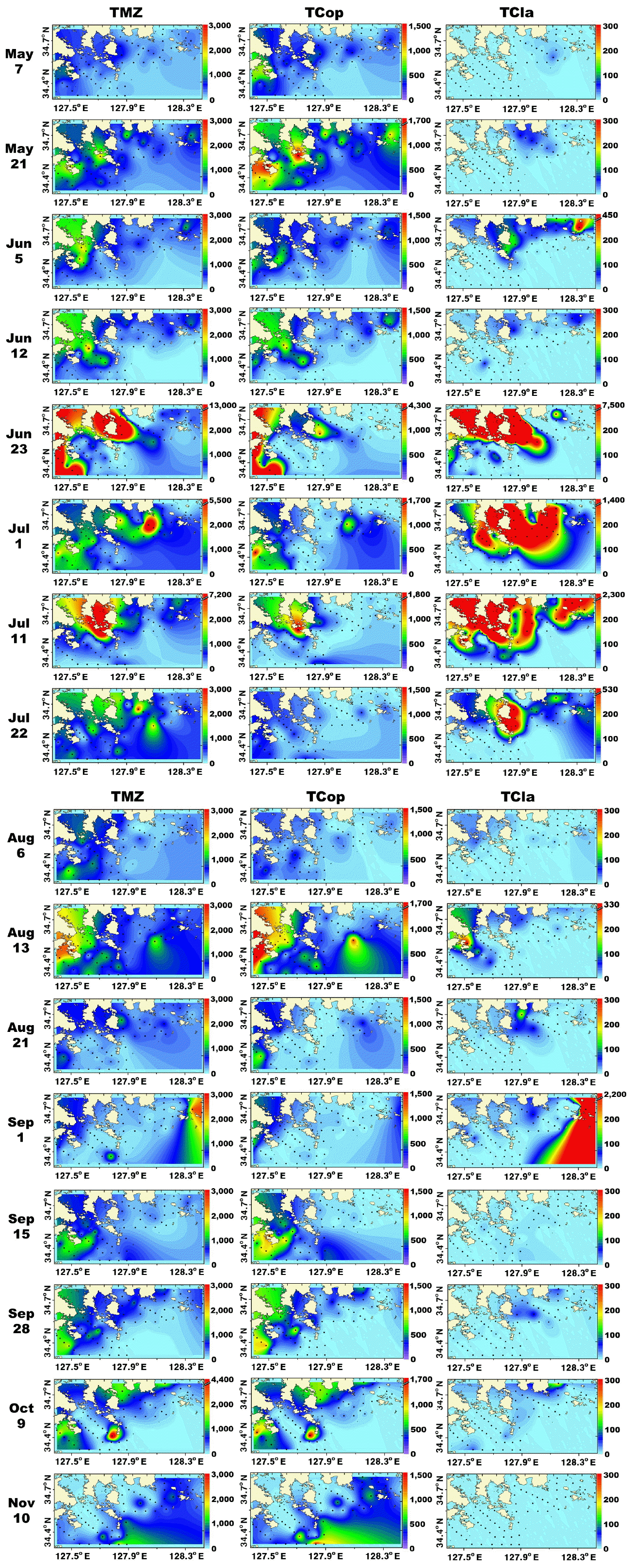
Fig. 5Distributions (inds. m−3) of larvae of invertebrates (LIV), chaetognaths (CHA), and hydrozoans (HYD) in the study area for each cruise from May 7 to Nov 10, 2014. 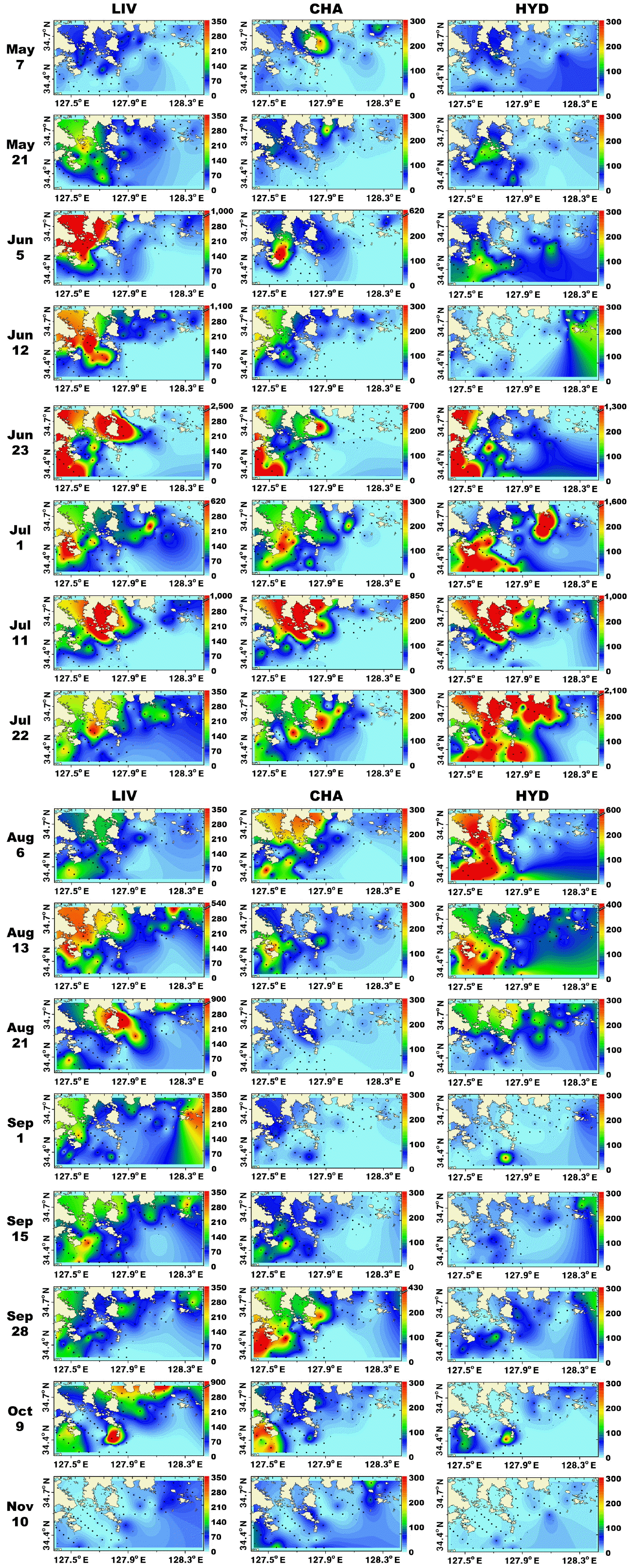
Fig. 6Calculated grazing coefficients (g, d−1) attributable to calanoid copepods on co-occurring Cochlodinium polykrikoides, based on assumption (see text) in the study area for each cruise from May 7 to Nov 10, 2014. 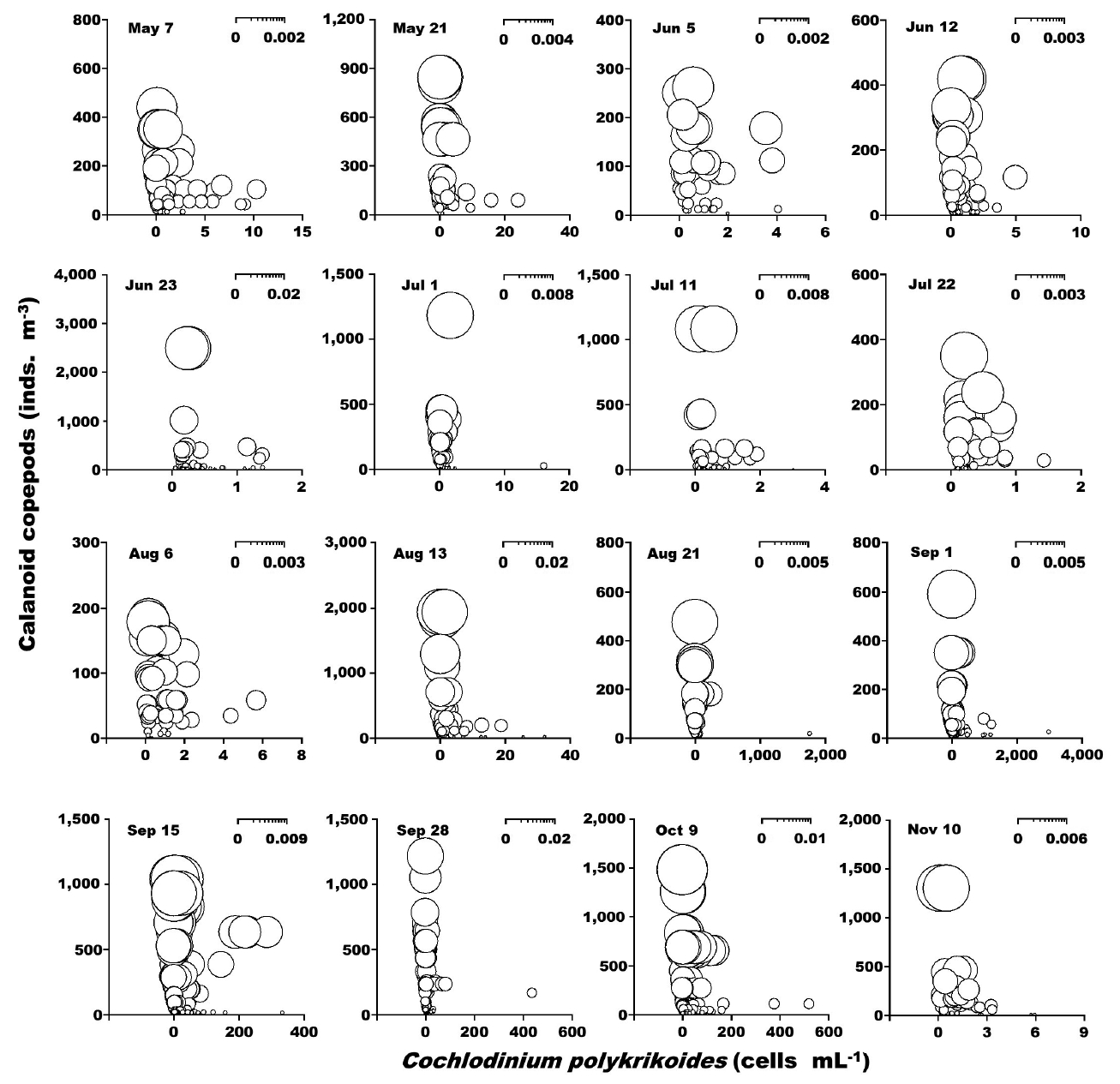
Fig. 7Calculated grazing coefficients (g, d−1) attributable to calanoid copepods on co-occurring Prorocentrum spp., based on assumption (see text) in the study area at each cruise from May 7 to Nov 10, 2014. 
Fig. 8Calculated grazing coefficients (g, d−1) attributable to calanoid copepods on co-occurring heterotrophic dinoflagellates Gyrodinium spp., based on assumption (see text) in the study area for each cruise from May 7 to Nov 10, 2014. 
Fig. 9Calculated grazing coefficients (g, d−1) attributable to calanoid copepods on co-occurring heterotrophic dinoflagellates Polykrikos spp., based on assumption (see text) in the study area for each cruise from May 7 to Nov 10, 2014. 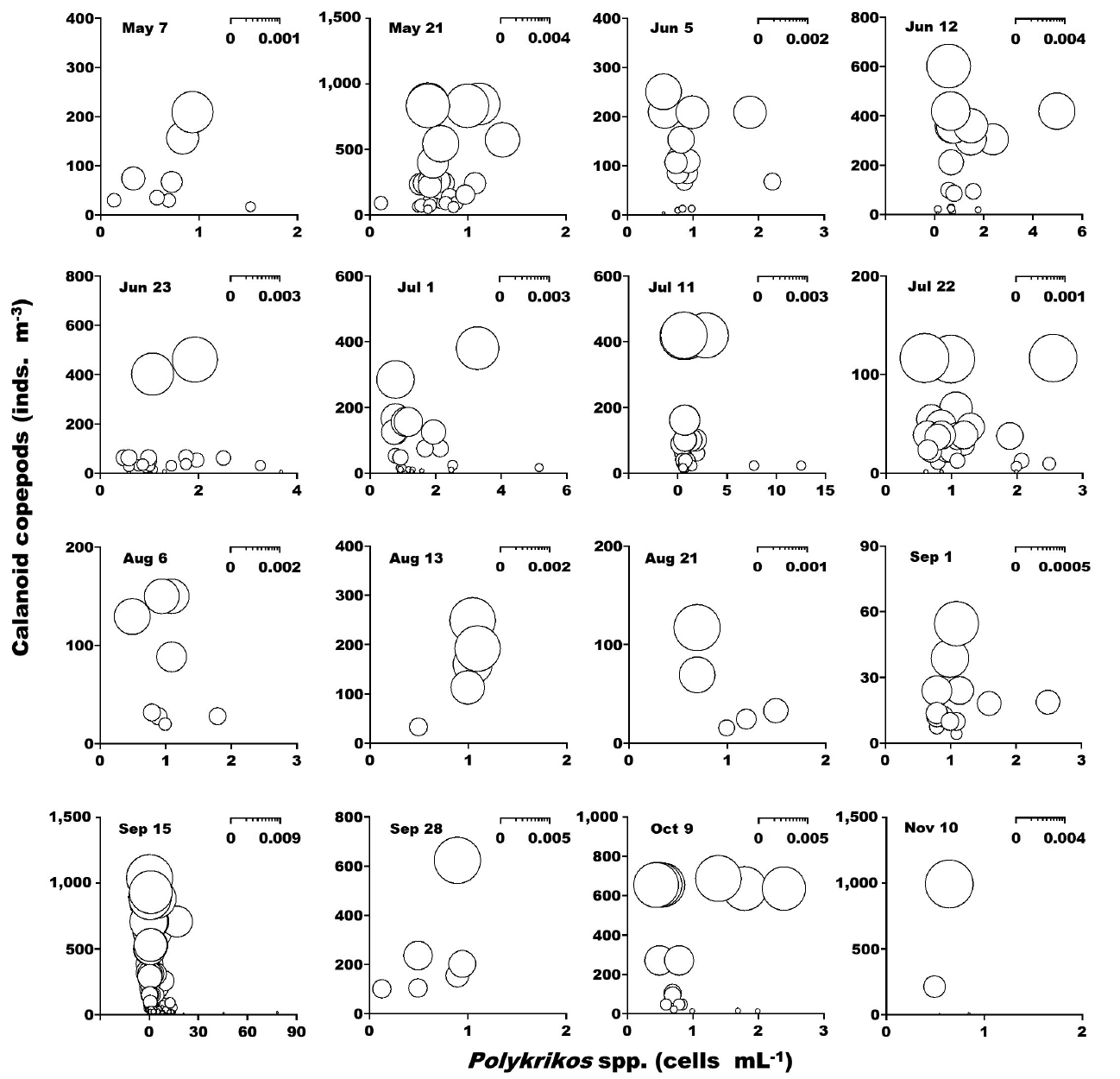
Table 1The range of abundance of total metazooplankton and each taxon, and water temperatures and salinity where each taxon was found in the South Sea of Korea from May to November 2014
a See Appendix 1. Table 2Correlations between the abundance of metazooplankton taxon and physical, chemical, and biological factors in the South Sea of Korea from May to November 2014
T, temperature; S, salinity; DO, dissolved oxygen; NO3, nitrite + nitrate; PO4, phosphate; SiO2, silicate; Chl-a, chlorophyll-a; DIA, diatoms; PTD, phototrophic dinoflagellates; EUG, eunglenophytes; CRY, cryptophytes; HTD, heterotrophic dinoflagellates; TCI, tintinnid ciliates; NCI, naked ciliates. a See Appendix 1. Table 3Correlations between the abundance of metazooplankton taxa and the abundance of potential diatom prey species in the South Sea of Korea from May to November 2014
DIA, diatoms; Caf, Chaetoceros affinis; Cco, Chaetoceros compressus; Ccu, Chaetoceros curvisetus; Cde, Chaetoceros decipiens; Cso, Chaetoceros socialis; Ezo, Eucampia zodiacus; Lbo, Lauderia borealis; Lda, Leptocylindrus danicus; Lme, Leptocylindrus mediterraneus; Lmi, Leptocylindrus minimus; Ppu, Pseudo-nitzschia pungens; Psp, Pseudo-nitzschia sp.; Sco, Skeletonema costatum; Tni, Thalassionema nitzchioides; Tde, Thalssiosira decipiens. Table 4Correlations between the abundance of metazooplankton taxa and the abundance of potential phototrophic dinoflagellate prey species in the South Sea of Korea from May to November 2014
PTD, phototrophic dinoflagellates; Asa, Akashiwo sanguinea; Asp, Alexandrium sp.; Cpo, Cochlodinium polykrikoides; Cfu, Ceratium furca; Cfs, Ceratium fusus; Cko, Ceratium kofoidii; Ctr, Ceratium tripos; Dca, Dinophysis caudata; Gsp, Gonyaulax sp.; Pba, Prorocentrum balticum; Pdo, Prorocentrum donghaiense; Pmi, Prorocentrum minimum; Ptr, Prorocentrum triestinum; Str, Scrippsiella trochoidea; Sym, Symbiodinium sp. Table 5Correlations between the abundance of metazooplankton taxa and the abundance of potential prey species in the South Sea of Korea from May to November 2014
EUG, eunglenophytes; CRY, cryptophytes; HTD, heterotrophic dinoflagellates; TCI, tintinnid ciliates; NCI, naked ciliates; Mru, Mesodinium rubrum; Nsc, Noctiluca scintillans; Pko, Polykrikos kofoidii; Ppa, Protoperidinium parvum; Prsp, Protoperidinium sp.; Tfu, Tiarina fusus; Ttu, Tintinnopsis tubulosoides. Table 6Maximum abundance (MA) of total metazooplankton and copepods and chlorophyll-a concentration where MA was obtained
REFERENCESAktan, Y. & Keskin, Ç. 2017. Second habitat record of Polykrikos hartmannii W. Zimm. (Dinophyceae) in the South Aegean Sea, Eastern Mediterranean. Turk J Fish Aquatic Sci. 17:1077–1081.
Anderson, DM., Alpermann, TJ., Cembella, AD., Collos, Y., Masseret, E. & Montresor, M. 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 14:10–35.
American Public Health Association (APHA) 1995. Standard methods for the examination of water and wastewater. 19th ed. APHA, Washington, DC, 1100 pp.
Calbet, A. 2001. Metazooplankton grazing effect on primary production: a global comparative analysis in marine ecosystems. Limnol Oceanogr. 46:1824–1830.
Calbet, A., Garrido, S., Saiz, E., Alcaraz, M. & Duarte, CM. 2001. Annual zooplankton succession in coastal NW Mediterranean waters: the importance of the smaller size fractions. J Plankton Res. 23:319–331.
Calbet, A., Vaqué, D., Felipe, J., Vila, M., Sala, MM., Alcaraz, M. & Estrada, M. 2003. Relative grazing impact of microzooplankton and metazooplankton on a bloom of the toxic dinoflagellate Alexandrium minutum
. Mar Ecol Prog Ser. 259:303–309.
Carlsson, P., Granéli, E., Tester, P. & Boni, L. 1995. Influences of riverine humic substances on bacteria, protozoa, phytoplankton, and copepods in a coastal plankton community. Mar Ecol Prog Ser. 127:213–221.
Cohen, JH., Tester, PA. & Forward, RB. Jr 2007. Sublethal effects of the toxic dinoflagellate Karenia brevis on marine copepod behavior. J Plankton Res. 29:301–315.
Conover, WJ. 1980. Practical nonparametric statistics. 2nd ed. John Wiley and Sons, New York, NY, 493 pp.
Croll, DA., Marinovic, B., Benson, S., Chavez, FP., Black, N., Ternullo, R. & Tershy, BR. 2005. From wind to whales: trophic links in a coastal upwelling system. Mar Ecol Prog Ser. 289:117–130.
Fu, FX., Tatters, AO. & Hutchins, DA. 2012. Global change and the future of harmful algal blooms in the ocean. Mar Ecol Prog Ser. 470:207–233.
Gallienne, CP. & Robins, DB. 2001. Is Oithona the most important copepod in the world’s oceans? J. Plankton Res. 23:1421–1432.
Glibert, PM., Anderson, DM., Gentien, P., Granéli, E. & Sellner, KG. 2005. The global, complex phenomena of harmful algal blooms. Oceanography. 18:136–147.
Griffin, SL., Herzfeld, M. & Hamilton, DP. 2001. Modelling the impact of zooplankton grazing on phytoplankton biomass during a dinoflagellate bloom in the Swan River Estuary, Western Australia. Ecol Eng. 16:373–394.
Hallegraeff, GM. 1993. A review of harmful algal blooms and their apparent global increase. Phycologia. 32:79–99.
Hansen, PJ., Bj⊘rnsen, PK. & Hansen, BW. 1997. Zooplankton grazing and growth: scaling within the 2–2,000-μm body size range. Limnol Oceanogr. 42:687–704.
Holmes, RW., Williams, PM. & Eppley, RW. 1967. Red water in La Jolla Bay, 1964–1966. Limnol Oceanogr. 12:503–512.
Houde, SEL. & Roman, MR. 1987. Effects of food quality on the functional ingestion response of the copepod Acartia tonsa
. Mar Ecol Prog Ser. 40:69–77.
Hue, HK., Kim, DH. & Ahn, SH. 2002. Community structure and distributions of zooplankton in Gangjin Bay in 1999. Koean J Environ Biol. 20:46–54.
Jansen, S., Riser, CW., Wassmann, P. & Bathmann, U. 2006. Copepod feeding behaviour and egg production during a dinoflagellate bloom in the North Sea. Harmful Algae. 5:102–112.
Jeong, HJ. 1999. The ecological roles of heterotrophic dinoflagellates in marine planktonic community. J Eukaryot Microbiol. 46:390–396.
Jeong, HJ., Kang, H., Shim, JH., Park, JK., Kim, JS., Song, JY. & Choi, HJ. 2001. Interactions among the toxic dinoflagellate Amphidinium carterae, the heterotrophic dinoflagellate Oxyrrhis marina, and the calanoid copepods Acartia spp. Mar Ecol Prog Ser. 218:77–86.
Jeong, HJ., Kim, JS., Song, JY., Kim, JH., Kim, TH., Kim, SK. & Kang, NS. 2007. Feeding by heterotrophic protists and copepods on the heterotrophic dinoflagellates Pfiesteria piscicida, Stoeckeria algicida, and Luciella masanensis
. Mar Ecol Prog Ser. 349:199–211.
Jeong, HJ., Kim, JS., Yoo, YD., Kim, ST., Kim, TH., Park, MG., Lee, CH., Seong, KA., Kang, NS. & Shim, JH. 2003. Feeding by the heterotrophic dinoflagellate Oxyrrhis marina on the red-tide raphidophyte Heterosigma akashiwo: a potential biological method to control red tides using mass-cultured grazers. J Eukaryot Microbiol. 50:274–282.
Jeong, HJ., Kim, JS., Yoo, YD., Kim, ST., Song, JY., Kim, TH., Seong, KA., Kang, NS., Kim, MS., Kim, JH., Kim, S., Ryu, J., Lee, HM. & Yih, WH. 2008. Control of the harmful alga Cochlodinium polykrikoides by the naked ciliate Strombidinopsis jeokjo in mesocosm enclosures. Harmful Algae. 7:368–377.
Jeong, HJ. & Latz, MI. 1994. Growth and grazing rates of the heterotrophic dinoflagellate Protoperidinium spp. on red tide dinoflagellates. Mar Ecol Prog Ser. 106:173–185.
Jeong, HJ., Lim, AS., Franks, PJS., Lee, KH., Kim, JH., Kang, NS., Lee, MJ., Jang, SH., Lee, SY., Yoon, EY., Park, JY., Yoo, YD., Seong, KA., Kwon, JE. & Jang, TY. 2015. A hierarchy of conceptual models of red-tides generation: nutrition, behavior, and biological interactions. Harmful Algae. 47:97–115.
Jeong, HJ., Lim, AS., Lee, K., Lee, MJ., Seong, KA., Kang, NS., Jang, SH., Lee, KH., Lee, SY., Kim, MO., Kim, JH., Kwon, JE., Kang, HC., Kim, JS., Yih, W., Shin, K., Jang, PK., Ryu, JH., Kim, SY., Park, JY. & Kim, KW. 2017. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: I. Temporal variations in three-dimensional distributions of red-tide organisms and environmental factors. Algae. 32:101–130.
Jeong, HJ., Shim, JH., Lee, CW., Kim, JS. & Koh, SM. 1999. Growth and grazing rates of the marine planktonic ciliate Strombidinopsis sp. on red-tide and toxic dinoflagellates. J Eukaryot Microbiol. 46:69–76.
Jeong, HJ., Yoo, YD., Kim, JS., Kim, TH., Kim, JH., Kang, NS. & Yih, W. 2004. Mixotrophy in the phototrophic harmful alga Cochlodinium polykrikoides (Dinophycean): prey species, the effects of prey concentration, and grazing impact. J Eukaryot Microbiol. 51:563–569.
Jeong, HJ., Yoo, YD., Kim, JS., Seong, KA., Kang, NS. & Kim, TH. 2010. Growth, feeding, and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sci J. 45:65–91.
Jeong, HJ., Yoo, YD., Lee, KH., Kim, TH., Seong, KA., Kang, NS., Lee, SY., Kim, JS., Kim, S. & Yih, WH. 2013. Red tidess in Masan Bay, Korea in 2004–2005: I. Daily variations in the abundance of red-tides organisms and environmental factors. Harmful Algae. 30(Suppl 1):S75–S88.
Kang, NS., Lee, KH., Jeong, HJ., Yoo, YD., Seong, KA., Potvin, É., Hwang, YJ. & Yoon, EY. 2013. Red tides in Shiwha Bay, western Korea: a huge dike and tidal power plant established in a semi-enclosed embayment system. Harmful Algae. 30(Suppl 1):S114–S130.
Kang, YS., Park, JS., Lee, SS., Kim, HG. & Lee, PY. 1996. Zooplankton community and distributions of copepods in relation to eutrophic evaluation in Chinhae Bay. Korean J Fish Aquat Sci. 29:415–430.
Katechakis, A. & Stibor, H. 2004. Feeding selectivities of the marine cladocerans Penilia avirostris, Podon intermedius and Evadne nordmanni
. Mar Biol. 145:529–539.
Kim, DI., Matsuyama, Y., Nagasoe, S., Yamaguchi, M., Yoon, YH., Oshima, Y., Imada, N. & Honjo, T. 2004. Effects of temperature, salinity and irradiance on the growth of the harmful red tide dinoflagellate Cochlodinium polykrikoides Margalef (Dinophyceae). J Plankton Res. 26:61–66.
Kim, JS. & Jeong, HJ. 2004. Feeding by the heterotrophic dinoflagellates Gyrodinium dominans and G. spirale on the red-tides dinoflagellate Prorocentrum minimum
. Mar Ecol Prog Ser. 280:85–94.
Kim, JS., Jeong, HJ., Yoo, YD., Kang, NS., Kim, SK., Song, JY., Lee, MJ., Kim, ST., Kang, JH., Seong, KA. & Yih, WH. 2013a. Red tides in Masan Bay, Korea, in 2004–2005: III. Daily variations in the abundance of metazooplankton and their grazing impacts on red-tides organisms. Harmful Algae. 30(Suppl):S102–S113.
Kim, MJ., Youn, SH., Kim, JY. & Oh, CW. 2013b. Feeding characteristics of the Japanese anchovy, Engraulis japonicus according to the distribution of zooplankton in the coastal waters of southern Korea. Korean J Environ Biol. 31:275–287.
Kim, ST. 2005. The interactions between dominant copepod Acartia spp. and red-tides organisms and protozoans in the coastal waters in the west and south coast in Korea, egg production rates and grazing impact in the coastal waters off the Saemankeum and Kwangyang Bay. Kunsan National University, Gunsan, 157 pp.
Kimmel, DG. & Roman, MR. 2004. Long-term trends in mesozooplankton abundance in Chesapeake Bay, USA: influence of freshwater input. Mar Ecol Prog Ser. 267:71–83.
Lazareva, VI. & Kopylov, AI. 2011. Zooplankton productivity at the height of eutrophication of a plain reservoir ecosystem: the role of invertebrate predators. Usp Sovrem Biol. 131:300–310.
Lee, MJ., Jeong, HJ., Lee, KH., Jang, SH., Kim, JH. & Kim, KY. 2015. Mixotrophy in the nematocyst–taeniocyst complex-bearing phototrophic dinoflagellate Polykrikos hartmannii
. Harmful Algae. 49:124–134.
Lim, AS., Jeong, HJ., Jang, TY., Jang, SH. & Franks, PJS. 2014. Inhibition of growth rate and swimming speed of the harmful dinoflagellate Cochlodinium polykrikoides by diatoms: implications for red tide formation. Harmful Algae. 37:53–61.
Lim, AS., Jeong, HJ., Seong, KA., Lee, MJ., Kang, NS., Jang, SH., Lee, KH., Park, JY., Jang, TY. & Yoo, YD. 2017. Ichthyotoxic Cochlodinium polykrikoides red tides offshore in the South Sea, Korea in 2014: II. Heterotrophic protists and their grazing impacts on red-tide organisms. Algae. 32:199–222.
Oh, HJ., Moon, SY. & Soh, HY. 2013. Seasonal changes of zooplankton communities along the coast of Geumo Archipelago, Yeosu. Korean J Environ Biol. 31:192–203.
Oh, SJ., Yoon, YH., Kim, DI., Shimasaki, Y., Oshima, Y. & Honjo, T. 2006. Effects of light quantity and quality on the growth of the harmful dinoflagellate, Cochlodinium polykrikoides Margalef (Dinophyceae). Algae. 21:311–316.
Park, TG., Lim, WA., Park, YT., Lee, CK. & Jeong, HJ. 2013. Economic impact, management and mitigation of red tidess in Korea. Harmful Algae. 30(Suppl 1):S131–S143.
Porter, KG., Sherr, EB., Sherr, BF., Pace, M. & Sanders, RW. 1985. Protozoa in planktonic food webs. J Eukaryot Microbiol. 32:409–415.
Puelles, MF., Grás, D. & Hernández-León, S. 2003. Annual cycle of zooplankton biomass, abundance and species composition in the neritic area of the Balearic Sea, Western Mediterranean. Mar Ecol. 24:123–139.
Sanders, RW. & Wickham, SA. 1993. Planktonic protozoa and metazoan: predation, food quality and population control. Mar Microb Food Webs. 7:197–223.
Smayda, TJ. 1990. Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. RWS-North Sea Directorate, Rijswijk, 29–40.
Sordo, I., Barton, ED., Cotos, JM. & Pazos, Y. 2001. An inshore poleward current in the NW of the Iberian Peninsula detected from satellite images, and its relation with G. catenatum and D. acuminata blooms in the Galican Rias. Estuar Coast Shelf Sci. 53:787–799.
Stoecker, DK. & Capuzzo, JM. 1990. Predation on protozoa, its importance to zooplankton. J Plankton Res. 12:891–908.
Tan, Y., Huang, L., Chen, Q. & Huang, X. 2004. Seasonal variation in zooplankton composition and grazing impact on phytoplankton standing stock in the Pearl River Estuary, China. Cont Shelf Res. 24:1949–1968.
Tillmann, U. 2004. Interactions between planktonic microalgae and protozoan grazers. J Eukaryot Microbiol. 51:156–168.
Tseng, LC., Dahms, HU., Chen, QC. & Hwang, JS. 2009. Copepod feeding study in the upper layer of the tropical South China Sea. Helgol Mar Res. 62:327–337.
Turner, JT. 2004. The importance of small planktonic copepods and their roles in pelagic marine food webs. Zool Stud. 43:255–266.
Turner, JT. & Borkman, DG. 2005. Impact of zooplankton grazing on Alexandrium blooms in the offshore Gulf of Maine. Deep Sea Res Part II Top Stud Oceanogr. 52:2801–2816.
Turner, JT. & Granéli, E. 1992. Zooplankton feeding ecology: grazing during enclosure studies of phytoplankton blooms from the west coast of Sweden. J Exp Mar Biol Ecol. 157:19–31.
Turner, JT. & Tester, PA. 1997. Toxic marine phytoplankton, zooplankton grazers, and pelagic food webs. Limnol Oceanogr. 42:1203–1213.
Turner, JT., Tester, PA. & Ferguson, RL. 1988. The marine cladoceran Penilia avirostris and the “microbial loop” of pelagic food webs. Limnol Oceanogr. 33:245–255.
Uye, S. 1986. Impact of copepod grazing on the red-tides flagellate Chattonella antiqua
. Mar Biol. 92:35–43.
Uye, S. & Liang, D. 1998. Copepods attain high abundance, biomass and production in the absence of large predators but suffer cannibalistic loss. J Mar Syst. 15:495–501.
Waggett, RJ., Tester, PA. & Place, AR. 2008. Anti-grazing properties of the toxic dinoflagellate Karlodinium veneficum during predator–prey interactions with the copepod Acartia tonsa
. Mar Ecol Prog Ser. 366:31–42.
Yoo, YD., Jeong, HJ., Kang, NS., Kim, JS., Kim, TH. & Yoon, EY. 2010. Ecology of Gymnodinium aureolum. II. Predation by common heterotrophic dinoflagellates and a ciliate. Aqut Microb Ecol. 59:257–272.
Yoo, YD., Jeong, HJ., Kim, JS., Kim, TH., Kim, JH., Seong, KA., Lee, SH., Kang, NS., Park, JW., Park, J., Yoon, EY. & Yih, WH. 2013a. Red tides in Masan Bay, Korea in 2004–2005: II. Daily variations in the abundance of heterotrophic protists and their grazing impact on red-tide organisms. Harmful Algae. 30(Suppl 1):S89–S101.
Yoo, YD., Seong, KA., Myung, G., Kim, HS., Jeong, HJ., Palenik, B. & Yih, W. 2015. Ingestion of the unicellular cyanobacterium Synechococcus by the mixotrophic red tide ciliate Mesodinium rubrum
. Algae. 30:281–290.
Yoo, YD., Yoon, EY., Jeong, HJ., Lee, KH., Hwang, YJ., Seong, KA., Kim, JS. & Park, JY. 2013b. The newly described heterotrophic dinoflagellate Gyrodinium moestrupii, an effective protistan grazer of toxic dinoflagellates. J Eukaryot Microbiol. 60:13–24.
Youn, SH., Oh, GS. & Chung, MH. 2010. Zooplankton community structure and copepod production in the Seomjin River Estuary. J Korean Soc Mar Environ Saf. 16:369–379.
Zar, JH. 1999. Biostatistical analysis. 4th ed. Prentice Hall, Upper Saddle River, NJ, 663 pp.
AppendicesAppendix 1.List of metazooplankton species and taxa present in the South Sea of Korea from May to November 2014 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||